Danielle Da Costa Leite Borges
Abstract
This article investigates policy and bureaucracy changes provoked by individual litigation for health care rights in Brazil, especially the one regarding access to medicines, looking at the effects it produced in relation to health technology assessment (HTA) and health care governance. The article first contextualizes the social, legal, and political conditions for the development of individual litigation for health care rights in Brazil. Then it points out the changes brought about by this litigation model and discusses their potential to contribute to efficiency and fairness in the health care system by the improvement of the HTA decision-making process and health care governance.
Introduction
The legal enforcement of health care rights may take a multitude of forms, ranging from orders to provide a specific medicine or treatment to a particular individual or group, to broad structural decisions declaring a particular state of affairs unconstitutional or even ordering the structuring of health services in a certain geographical area.[1] David Landau, for example, identifies four remedial forms: a) individual enforcement; b) negative injunctions; c) weak-form enforcement; and d) structural enforcement.[2] According to the first one, courts grant rights to a single plaintiff, such as the provision of a medication or treatment. The negative injunction model is often used to strike down benefit cuts or other laws that diminish social benefits. With weak-enforcement, also called the dialogical model, courts point out political failures to uphold social rights but leave the remedy at the discretion of political branches. Finally, structural enforcement occurs when courts issue broad orders aimed at (re)structuring institutional or policy practices.
Courts in developing countries have relied mainly on two broad models of social rights enforcement: the individual model and the negative injunction model.[3] In Brazil, thus far, the most prevalent form of enforcement has been the individual model, especially when it comes to access to medicines claims, although in recent years the country has also experimented with the use of the structural model.[4] This way of litigating for health care rights, also found in other Latin American countries, consists of lawsuits brought mostly by individual plaintiffs represented by private or public attorneys (the latter in the case of plaintiffs with earnings below a certain threshold, which varies across the country) against public authorities—states, municipalities, or the federal government—claiming mostly the provision of a specific medication or treatment and encountering a very high success rate in the courts.[5] The effects of these decisions apply only inter partes, that is, between the parties of the case.
The threshold to win in the courts is very low, insofar as the individual litigant must simply prove that a health need (access to medication or treatment), as described in a doctor’s prescription, was not met. Therefore, in the Brazilian model of litigation, the doctor’s prescription (from a state or private health facility) is very often the only relevant document necessary for a court to render a decision imposing on the state the obligation to provide a particular medication or treatment to a particular individual.[6]
Another reason explaining the prevalence of this model of litigation in Brazil is the fact that Brazilian courts are more open to individual claims than collective ones, creating a strong incentive for plaintiffs to bring forth individual action.[7] Collective claims have a much more far-reaching impact than individual ones because the effects of their decisions apply erga omnes. They are usually brought by the prosecutor’s office (Ministério Público) through a legal procedure called ação civil pública and concern public authorities’ failure to comply with legal obligations in guiding structural health policies. Brazil has also seen a slight increase in collective litigation through public class actions aimed at implementing health policies, however they are still low in numbers compared to individual claims.[8] Experienced structural litigation since the prevalent litigation strategy used in health care cases in the country is the individual one. For instance, only 2% of the 7,400 health cases of Florian and Bentes’ database are collective cases. Therefore, individual solutions tend to take precedence over structural orders.[9]
It is worth noting that, apart from the public (and universal) health care system in Brazil, there is also a parallel, private system of care. People using the private health care system are usually wealthier individuals who can afford private health insurance, or employees who have health insurance as part of their benefits package. However, lawsuits concerning the provision of medication can be brought against public authorities despite the fact that the individual is insured privately. Indeed, according to Brazilian law, private health insurers only have to provide medication in case of inpatient treatment. The only exception to this rule regards some anti-cancer drugs for outpatient cancer treatment.[10] Moreover, cases concerning the private provision of health care are mostly ruled according to private law—contract and consumer laws—whereas cases regarding the public provision of health care, such as the ones discussed in this article, are decided exclusively according to constitutional law. Therefore, many health insured individuals resort to the public health system to access medicines that they would not be allowed to through private health insurance. Still, there is also a high number of individual claims brought against private health insurers in Brazil. These claims regard contract coverage, contract breach, health treatments, and only a residual part refers to medicines.[11]
The frequent use of individual litigation to enforce the right to health has been the subject of intense debate in the literature. Some have criticized it for rendering the public health system less fair and rational, since the often sole criterion used by courts to grant claims (individualized medical prescriptions) disregards the need to set priorities according to sound public health reasons. Moreover, given important difficulties of access to justice in Brazil, it can often favor those who are financially equipped to hire private lawyers or access the limited provision of state attorneys.[12] Therefore, judicialization could widen the social gap in Brazil, diverting public resources from the most deprived individuals and from other important areas of health. However, others have contested these conclusions and have argued that “judicialization may serve as a grassroots instrument for the poor to hold the state accountable.”[13]
In this article, however, I do not focus on these debates. Here, I approach individual litigation for health care rights in Brazil from a different perspective, looking at the effects it produced in relation to health technology assessment (HTA) and health care governance. More than triggering bureaucratic changes, as I have maintained elsewhere, here I argue that individual litigation for health care rights in Brazil has pushed forward policy changes that ranges from strengthening health technology assessment processes to better health care governance through institutional dialogue between different state actors.[14] Accordingly, by looking specifically at the case of individual litigation related to access to medicines, I will show that, although focusing mostly on individual cases, this phenomenon has brought about structural changes that have the potential to produce positive effects in terms of efficiency and fairness in the Brazilian public health system.
In order to develop this argument, I will first provide a historic overview of the social and political contexts in which individual litigation developed in Brazil since the promulgation of the Brazilian constitution in 1988. Following this, I will describe the institutional changes produced by the health care-related individual model of litigation in Brazil, especially when it comes to health technology assessment and health care governance, presenting data available in these areas in order to demonstrate how these changes, although not directly related to social justice, can potentially contribute to the achievement of more fairness and efficiency in the Brazilian health system.
An historic overview of individual litigation for health care rights in Brazil
Health care-related individual litigation in Brazil developed in a favorable historical time frame by virtue of Brazil’s democratization process. It started in the mid-1990s, following Brazil’s constitutional milestone in 1988, which reinstated democracy and provided a set of constitutionalised rights, including the right to health, and coincided with the peak of the AIDS epidemic, which explains, therefore, the reason why most of the lawsuits then regarded HIV medication. Although the institutionalized health program for HIV in Brazil dates from 1986, it was only in 1996 that a federal law established the free distribution of medication throughout the country.[15] Apart from the legal framework, the mobilisation of civil society was another major driving force behind the then on-going process of constructing and implementing a policy of free access to HIV medication in Brazil.[16] Nevertheless, since not all antiretroviral drugs were encompassed by the Brazilian HIV therapeutic guidelines, patients started to claim in courts antiretroviral drugs that were not yet made available by the national program. For instance, in Rio de Janeiro, between 1991 and 1998, lawsuits concerning HIV medication corresponded to 90% of all lawsuits regarding access to medicines, whereas in the Supreme Court they corresponded to one third of health care-related litigation.[17]
Brazilian courts have been receptive to individual litigation since at least the late 1990s, granting in most of the cases the medication claimed by HIV patients.[18] From 1997 on, with the advancement and structuring of the Brazilian Program for AIDS, antiretrovirals started to be regularly dispensed by state health authorities, contributing to the fall of individual litigation in this area.[19] During these years, the government revealed itself to be committed to fighting the AIDS epidemic globally.[20] For instance, Brazil twice used the threat of compulsory license as a strategy to pressure drug companies into price negotiations for HIV medication and, in 2007, effectively issued a compulsory license for the antiretroviral Efavirenz.[21]
The favorable judicial environment found by patients during the 1990s led more people suffering from other diseases to claim medication before courts.[22] Currently, individual litigation concerns medication for a variety of diseases, ranging from rare diseases, such as Gaucher’s Disease and Duchenne muscular dystrophy, to chronic diseases, such as diabetes, cancer, hypertension, and hepatitis C.[23]
In addition to the favorable judicial environment (that is, the low threshold established by Brazilian courts), other problems also contributed to the increment of individual litigation for medicines.[24] This includes, for example: a) the underfunding of the health sector, whose budget between 2002 and 2008 corresponded to an average of 3.6% of the GDP; b) the difficulties of establishing a basis for organizing services at a much-decentralized health system (the country is divided into 26 states, one federal district and more than 5,570 municipalities which have administrative autonomy in terms of health policy implementation); and c) the fragmentation of pharmaceutical assistance policies.[25] Many of these managerial problems regarding pharmaceutical assistance are for instance described by health officers themselves in inspection reports issued by the Brazilian Federal Court of Auditors.[26]
Therefore, the number of individual claims in Brazilian courts has risen steadily since 2000. According to data available at the National Council of Justice, in 2014 there were about 62,291 lawsuits regarding medicines and treatments against the Federal Union, and about 330,603 against states, municipalities, and the federal district.[27] However, after 2009, new strategies and institutional changes were put in place in an attempt to control the number of lawsuits and public expenditure. These changes in bureaucracy at different levels and in different governmental institutions were already discussed by some authors, such as Wang, Ribeiro and Hartmann, and Duarte.[28] Although some of these authors are quite sceptical about such changes, in my view they have the potential to affect positively the Brazilian public health system, as they have established a more transparent health technology assessment process in the country and new forms of inter-institutional dialogue and of healthcare governance. This, in turn, may not only contribute to reducing individual litigation, but also to advancing efficiency and fairness in the Brazilian health system, as I will discuss in the following sections.
Bureaucratic changes after 2009: Strengthening HTA and new forms of health care governance
In March 2009, acknowledging, on the one hand, the several cases pending before the Brazilian Supreme Court (STF) regarding individual litigation for the supply of medication, and, on the other hand, the limited institutional capacity of judicial power to alone deal with technical issues arising from these cases, the then-president of the STF, Justice Gilmar Mendes, convened health authorities and experts in the health field at a public hearing in order to clarify technical, scientific, administrative, political, and economic issues surrounding health care provision. During the opening of the public hearing, Justice Mendes declared that he expected the event not only to feed the court with technical information, but also to promote a broader and pluralist debate for the improvement of health policies.[29]
The main outcome of the public hearing was the establishment of criteria to guide the Court on future and pending decisions on health care cases. In effect, in March 2010, the STF ruled on nine cases, establishing non-binding guidelines for how courts should deal with medicines claims from then on. Santos and colleagues, who analysed the STF public hearing in light of the social systems theory from Niklas Luhmann, concluded that it proved to be strategic insofar “there was a mutual learning between the political and legal systems by structural coupling of such public hearing.”[30] Moreover, the legal system incorporated important arguments discussed during the public hearing, such as the one on the rejection of legal requests for unregistered drugs before the National Health Surveillance Agency (ANVISA).[31]
In terms of institutional effects, the public hearing can be seen as a first formal step towards a constitutional dialogue between the executive and the judiciary branches of the government, as it triggered a sequel of communications between different state actors with the shared intention of improving the practice of interpreting the constitution.[32] Moreover, it demonstrates the judiciary’s potential for enhancing democracy and participation in a practical example of dialogic justice.[33]
In this regard, after the public hearing, and also as a response to the number of health care-related lawsuits pending at Brazilian courts, other important changes have taken place at the judiciary level. These include the creation of a working group by the National Council of Justice (CNJ)—which by this time was also under the presidency of Justice Gilmar Mendes—to study, propose measures and guidelines aimed at preventing health care-related litigation, and help the country’s tribunals in dealing with these cases.[34] In fact, the work developed by this group evolved, and in March 2010 the CNJ published a recommendation, providing some criteria to assist magistrates and other legal professionals to ensure greater efficiency in the settlement of health care-related lawsuits.[35] Following this, in April 2010, the CNJ established a permanent forum on health issues aimed at monitoring and finding solutions to health care litigation.[36] This forum meets frequently, and, for instance, in December 2017, the current president of CNJ, Justice Cármen Lúcia, convened a new public hearing where the actors involved in the problem of individual litigation discussed the current state of affairs, presented data, and shared best practices.[37]
While these initiatives developed at the highest level of the judicial branch, other changes at different levels of the legislative and executive branches of the government took place, affecting the health technology assessment process and health care governance as will be demonstrated.
The HTA process in Brazil
According to the World Health Organization (WHO), HTA refers to the systematic evaluation of properties, effects, and/or impacts of health technology. It is carried out through a multidisciplinary process which evaluates the social, economic, organizational and ethical issues of a health intervention or technology aiming to inform policy decision making.[38] It works as an important policy tool in the management of healthcare delivery in conditions of resource constraint and contributes to fostering health equity especially in developing and emerging countries.[39] However, the institutionalization of HTA in these countries is still considered immature, focusing mostly on training and instruction of personnel to perform HTA whereas this process also involves political commitment, capacity for investment, maturity of the decision-making process and the structure of national health systems.[40]
In Brazil, discussion about HTA began in 1983.[41] Although since 2000 there have been institutional changes aiming at establishing some kind of HTA process in the country, it was only in 2006 that this process was formally instituted through law with the establishment of the Commission for the Incorporation of Technologies (CITEC), which worked under the supervision of the Health Attention Secretariat of the Ministry of Health (Secretaria de Atenção à Saúde). In 2008, the Secretariat of Science, Technology, and Strategic Inputs (SCTIE) took over the role of coordinating and supervising the process of incorporation of new technologies. Under the auspices of SCTIE, the process flow was redefined and improved, with the establishment of deadlines and criteria for the submission of proposals and issuing of reports on the incorporation of technologies.[42]
But only in 2011, following the constitutional dialogue started at the Brazilian Supreme Court in the public hearing on judicialization discussed in the previous section, that a new federal law (Law n. 12.401/2012) was enacted, creating a new HTA body named CONITEC (National Committee for the Incorporation of Technologies in the Public Health System, or Comissão Nacional de Incorporação de Tecnologias no Sistema Único de Saúde) under the auspices of the Ministry of Health. CONITEC replaced CITEC and the main justification for its creation—according to the explanatory notes of the two draft bills that led to the adoption of law n. 12.401/2012—was the phenomenon of individual health care litigation.[43] In terms of its operational structure, CONITEC consists of two different boards: the executive secretariat and the plenary. The latter is responsible for issuing recommendations and consists of 13 members, with seven of them coming from different secretariats of the Ministry of Health and the other six from different institutions across the health system: the National Council of Municipal Health Secretaries (Conselho Nacional de Secretarias Municipais de Saúde, CONASEMS), the National Council of State Health Secretaries (Conselho Nacional de Secretários de Saúde—CONASS), the National Health Council (Conselho Nacional de Saúde—CNS), the National Regulatory Agency for Private Health Insurance and Plans (Agência Nacional de Saúde Suplementar—ANS), the National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária—ANVISA), and the Federal Council of Medicine (Conselho Federal de Medicina—CFM). The Executive Secretariat is in charge of managing and coordinating the activities of CONITEC, including the issuing of the final recommendation reports. Regarding its mission, CONITEC’s main competence is to provide technical advice to the Ministry of Health in decisions regarding the incorporation, exclusion or alteration of health technologies within the Brazilian health system, as well as in the formulation or modification of clinical protocols and therapeutic guidelines. According to Law 12.401/2012 and its accompanying decree (7.646/2011), this is to take effect through an administrative procedure that is open to public participation by means of public hearings and public consultation. Decisions made on these procedures are subject to administrative appeal from interested parties.
Recommendations take into account available scientific evidence regarding efficacy, effectiveness and safety of medicines, procedures and medical devices, as well as health economic evaluation and budget impact studies. In effect, CONITEC frequently cross-references HTA assessments made by other important international HTA agencies, such as the National Institute for Health and Clinical Excellence (NICE), the Canadian Agency for Drugs and Technologies in Health (CADTH), and the Australian Pharmaceutical Benefits Advisory Committee (PBAC).[44] In addition, due to CONITEC’s membership in the International Network of Agencies for Health Technology Assessment (INAHTA), CONITEC’s recommendations can benefit from shared information of the other 48 HTA agencies members of this network. In effect, another important attribution of CONITEC is to revise and update regularly the national list of essential medicines (RENAME).[45] Aiming at increased agility and efficiency, the process analysis of incorporation of technologies should take 180 days (extendable for another 90 days) and the full list of appraisals is regularly updated and made available at CONITEC’s website.[46]
Therefore, the creation of CONITEC brought substantial improvements to the institutionalization of HTA, especially as compared to the old decision-making process. Previously, appraisals were not publicly disclosed, there was no clear timeline for a review and decision-making after a positive recommendation, there were no public hearings or public consultations, and the right of appeal was much more restricted.[47] The new process has also improved productivity in terms of the number of appraisals issued per year; it has in fact tripled the number of appraisals in a year when compared to the previous decision-making process.[48] Furthermore, there is also some evidence that the quality of the decisions has been improved. For example, in a study on the relationship between the quality of evidences and CONITEC’s recommendation reports on medicines between 2012 and 2015, Zimmerman and colleagues concluded that these recommendations present consistent trends on the use of quality of evidences as well as on the use of economic and implementation aspects. Moreover, their analysis also shows that CONITEC’s recommendations use a multiple criteria analysis process, suggesting that they contribute to decisions in line with the Brazilian health system’s needs.[49] A similar analysis carried out by Caetano and colleagues, which investigated the decision-making process, profile of demands and incorporation of new medicines in the Brazilian public health system (Sistema Único de Saúde – SUS) from January 2012 to June 2016, suggests incremental rationality and the presence of clinical and economic evidence based on CONITEC’s decisions.[50]
Nevertheless, it is still difficult to assess other aspects regarding the impact of CONITEC over the Brazilian public health system, as there are still few studies on the substance of the decisions taken. Hence, further research is needed in this area in order to investigate other aspects, such as the scientific rigor, legitimacy, and independence of decisions.
With regard to health care-related litigation, CONITEC has established direct communication channels with the judiciary, which can send direct requests (via email) regarding information on a certain medication or health technology in order to subsidize judicial decisions. For instance, between 2014 and July 2017, CONITEC replied to around 1,500 requests emails sent by the judiciary.[51]
Moreover, there is some evidence that the new HTA process in Brazil, and thus the availability of technical decisions to the judiciary and administrative health authorities at the state level, have contributed to the decrease in health spending on health care individual litigation. In the state of São Paulo, for example, between 2013 and 2014 there was a decrease of around R$5 million (approximately US$1.5 million), reflecting a 1.5% reduction in health care spending with individual litigation at the state level. In relation to requests solved at the administrative level, that is, requests for medicines which are presented to local health authorities and solved at this level without the need to file a lawsuit, there has been a decrease of about R$150 million (approximately US$ 46 million) or 40% between 2012 and 2014.[52] The number of requests decreased by 25% between 2014 and 2015 (Graph 1).[53]
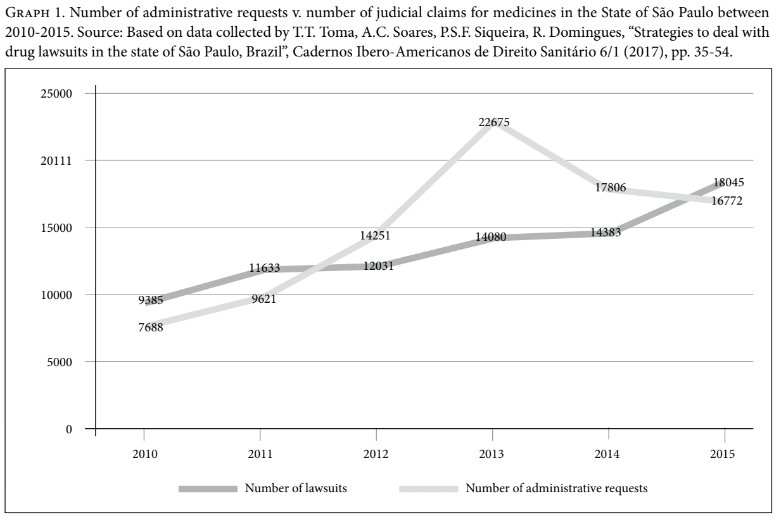
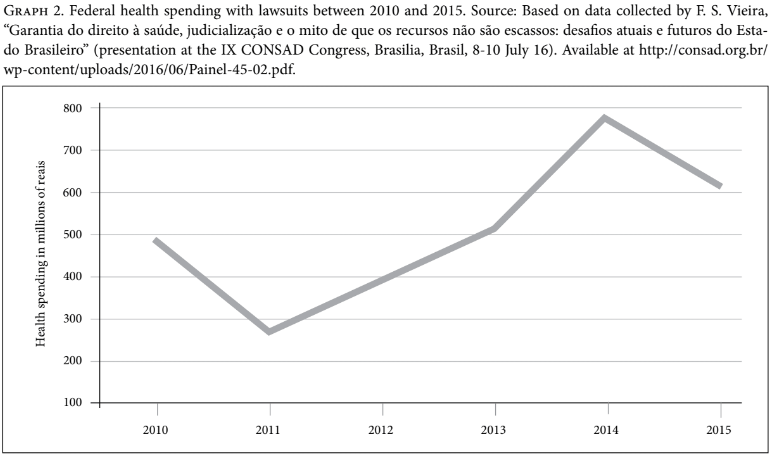
Furthermore, at the federal level, although there has been a substantial increase in health spending with medicines in the last seven years, spending on lawsuits decreased by 20% between 2014 and 2015 (Graph 2).[54] Further investigation is still needed in order to explain this decrease, but a possible explanation could be related to the incorporation by CONITEC of some of the most requested drugs by individual litigants. For example, Trastuzumab for breast cancer, Palivizumabe for respiratory syncytial virus, Rituximab for rheumatoid arthritis, and drugs for treating hepatitis C (Sofosbuvir, Simeprevir, and Daclastavir) were all incorporated between 2012 and 2014.[55]
Individual litigation therefore influenced the establishment of a more transparent, participatory, and accountable decision-making process regarding HTA in Brazil with the creation of CONITEC. This, in turn, can contribute to the advancement of fairness in the health system, as health technology assessment is considered an important tool in this regard. It not only sets more transparent rules and procedures for allocating health resources but also promotes fairness by making drugs available to the population at large and not only to individual claimants.
New strategies in health care governance: Collaborative governance and ‘de-judicialization’
Health governance can be defined as “a wide range of steering and rule-making related functions carried out by governments/decisions makers as they seek to achieve national health policy objectives that are conducive to universal health coverage.”[56] It is thus an important mechanism in establishing health policies aimed at increasing efficiency and fairness in health systems.
The phenomenon of individual litigation has challenged not only health authorities, but all actors involved in it, to finding new strategies to deal with and overcome this specific policy problem. The strategies adopted in Brazil to deal with individual litigation for health care affected, in my opinion, two elements of health governance: participation and capacity. According to Greer and colleagues, participation in health care governance means that affected parties have access to the decision-making process. One of the mechanisms to achieve participation is joint workforce, which works specifically in situations where the problem is the participation of different parts of government in a particular policy problem. The same authors refer to capacity, or policy capacity, as the ability to turn a political idea into a work proposal. Some of the mechanisms to improve it rely on intelligence on process (by, for example, understanding legal and budgetary issues that need to be changed) and specialist advice into policy formulation and recommendation.[57] As I will discuss in this section, the establishment of new institutional arrangements have improved participation and capacity advancing therefore health care governance in Brazil.
Since 2009, following the public hearing at the Brazilian Supreme Court, different actors involved in the phenomenon of health care individual litigation all over the country started to discuss policy improvements needed to overcome the problem. This involved, for example, state courts, local health authorities, state attorneys’ offices (Procuradorias do Estado e Município), public defenders’ offices (Defensoria Pública), public prosecutors’ offices (Ministério Público) and technical health experts. In this regard, one of the first initiatives was the one of the Rio de Janeiro State Tribunal (TJRJ). In February 2009, this state court and the local health authority signed a cooperation agreement regarding the implementation of an advisory health committee (Núcleo de Assessoria Técnica—NAT) to provide technical advice to the state tribunal in cases concerning the supply of medication and other medical goods. The committee consists of permanent civil servants of the state health authority in the field of medicine, nursing, pharmacy, nutrition, and management and has a consultative status. Its main mission is to give advice on medication and other judicially claimed medical goods. Accordingly, NAT’s advisors prepare appraisals considering objective and subjective aspects of lawsuits concerning the provision of medicines, such as: if the drug claimed is registered with the national surveillance agency (ANVISA), if it is part of the national list of essential medicines (RENAME) and if the drug requested is suitable for the treatment of the pathology in case, considering the claimant’s age and the amount requested.
According to Normative Act 5/2012, enacted by the Rio de Janeiro State Tribunal (TJRJ), all lawsuits concerning the provision of medicines or medical goods must be sent to NAT’s advisors, who should prepare appraisals within 48 hours after receiving information on a certain lawsuit.[58] Appraisals are prepared prior to any judicial decision. However, NAT’s appraisals are not binding on judges, due to the principle of the independence of the judiciary.
Despite the non-binding status of NAT’s technical appraisals, a qualitative study which investigated, among others, judges’ opinions on the relevance of NAT’s work, revealed that the idea of NAT is quite well accepted by magistrates, who feel more “safe” and “secure” to take decisions having these technical appraisals.[59] Nevertheless, due to the lack of other qualitative or quantitative studies on this topic, it is not possible to measure yet whether judges take into account these technical appraisals when deciding cases. Still, the establishment of NAT has brought advantages to the judgement of these lawsuits with regard to technical capacity, celerity and costs. Before the establishment of NAT, judges would either decide without a technical appraisal, relying only on the drug prescription presented, or would nominate a private technical advisor and commission an appraisal on the specific medicine claimed (perito do juízo). In this case, this is not only more costly, because private advisors charge for their appraisals, which are paid either by the parties or the judiciary (state), but it also takes much more time, insofar the procedure for technical appraisals established by the Brazilian Civil Procedural Code is much longer than the 48 hours within which NAT should present its appraisals (see Articles 464 to 480 of Law n. 13.105/2015).[60]
The 2010 CNJ Recommendation n. 31, among other recommendations, called state and federal tribunals to provide technical aid to judges in order to assist their decisions on lawsuits regarding the provision of medicines and/or medical goods by the establishment of consultative bodies such as NAT. Accordingly, consultative bodies similar to NAT have been implemented across the country and, as of March 2017, 17 other states had established such bodies: Rio Grande do Sul, Espírito Santo, Mato Grosso, Mato Grosso do Sul, Pernambuco, Piauí, Acre, Bahia, Goiás, Paraíba, Paraná, Santa Catarina, Tocantins, Minas Gerais, Pará, Rio Grande do Norte, and São Paulo.[61]
Although as yet there are no scientific studies evaluating the impact of these bodies on the phenomenon of judicialization, some health authorities have reported that they had a positive impact, contributing to a decrease in the number of lawsuits and health spending concerning the provision of health care-related items requested by individual litigants. For example, Rio Grande do Sul, one of the leading states in terms of number of health lawsuits in Brazil, reported that due to the work of the advisory health committee, between 2010 and 2017, there was a 35% decrease in the number of lawsuits, representing a 17% decrease in health spending.[62] Likewise, in the state of São Paulo between 2013 and 2014 a 1.5% decrease in health spending with individual litigation was observed (R$5 million or approximately US$1.5 million), as previously mentioned in this article.[63]
In 2013, NAT’s initiative gained a broader scope with the creation of a mediation and conciliation centre (Câmara de Resolução de Litígios de Saúde—CRLS) in the state of Rio de Janeiro. Devoted exclusively to health-related issues, the CRLS came about due to a partnership between the state attorney’s office (Procuradoria do Estado do Rio de Janeiro), the public defender’s and prosecutor’s offices in Rio de Janeiro, and local health authorities.
The CRLS works at a preventive or pre-judicial level, before the filing of a lawsuit, and aims at preventing litigation. Accordingly, when receiving medication requests from individuals, the local health authority either dispenses the item or, if this is rejected for some reason, the individual is redirected to the CRLS, which will set a first meeting with its social workers. They proceed to a “screening” of the case to check documents, including the prescription, and will send this data to the public defender and CRLS technical staff (medicine, nursing, and pharmacy professionals, for example, from the local health authority). This technical analysis aims at checking, for example, whether the drug requested is listed on the official lists, and, if not, if there is any substitute to the requested item in the national and local lists. If the item is part of the official lists, the local health authority simply issues the necessary documents for the collection of the medicine. When the medicine is not part of the lists, the individual is referred to a new medical visit with his own doctor or with one from a public facility, so that the doctor can inform whether the substitute medicine available is suitable for this individual. With a positive answer in this regard, the local health authority proceeds with the grant of the substitute item. In the case of negative feedback from the doctor, the health authority will assess the medical justification and decide whether or not to grant it. However, at any time, the individual or the public defender can opt to file a lawsuit.
Therefore, the CRLS opened a mediation channel which can prevent the file of new lawsuits. Before its creation, there was no structured process allowing for mediation at the administrative level or before the file of a lawsuit relating to health care-related items. Any conciliation would only take place at the judicial level, according to civil procedural rules, and would not count on the technical information provided by CRLS. Flow charts 1 and 2 represent the process flows with and without the participation of the CRLS.
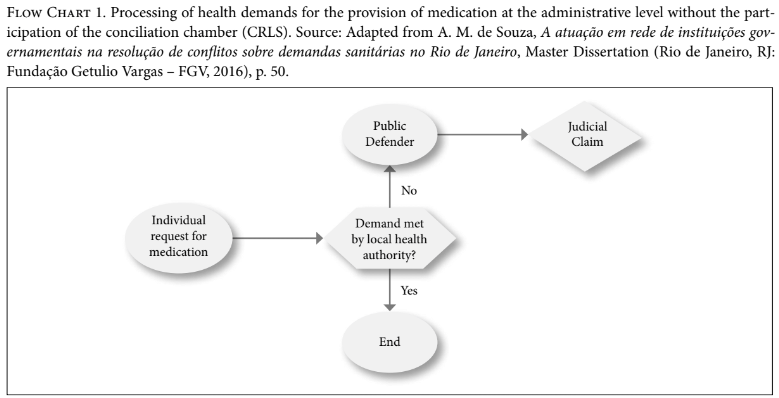
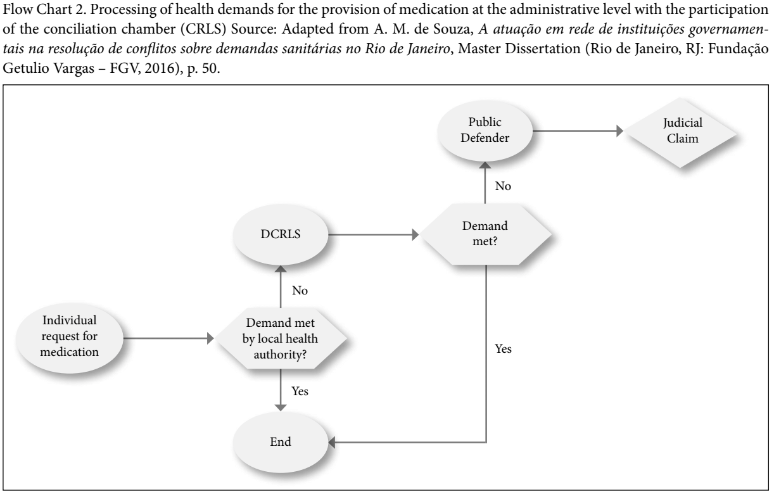
Due to the lack of scientific studies on the subject, it is currently not possible to assert whether the work of the CRLS has actually reduced the filing of new lawsuits. Indeed, the chamber’s activities have been in place for only a couple of years. However, the state attorney’s office in Rio de Janeiro claims that the establishment of CRLS has been avoiding the filing of new lawsuits. According to its data, between September and December 2013, 70 days after its establishment, there was a 38% decrease in the filing of new lawsuits.[64] This decrease trend remained constant after one year, when a 37.1% decrease was observed.[65] In terms of expenditure, the state attorney’s office and the local health authority estimate that between 2014 and 2015, it represented an R$11 million (approximately U$3.4 million) decrease in health spending with litigation for health care-related items. In absolute numbers, they calculate that after three years, the CRLS prevented filing of around 15,000 lawsuits.[66]
Similar models of mediation chambers have been adopted by other local authorities around Brazil, such as Distrito Federal and Bahia. The former established in February 2013 a conciliation chamber called Câmara Permanente Distrital de Mediação em Saúde – Camedis. Public authorities from these states have also released data that indicate positive results of these initiatives. Accordingly, in relation to Distrito Federal, it is estimated that around 85% of the requests submitted to Camedis were settled at the administrative level, resulting in a 20% decrease in the number of lawsuits filed between 2013 and 2015.[67] In relation to Bahia, local authorities and the CNJ have reported that the work of the mediation and conciliation centre for health issues settled around 80% of the cases received.[68]
Along the same lines, the state of São Paulo has in recent years adopted many initiatives at the administrative level aimed at avoiding new lawsuits. Local authorities have reported that due to these initiatives, the number of lawsuits has dropped for two consecutive years (2016 and 2017): 2% in the first year and 16% in the second, reflecting a decrease of approximately US$63 million in health spending with medication requested in individual lawsuits.[69] During its presentation at the public hearing in December 2017, the state health authority in São Paulo (Secretaria de Estado da Saúde do Governo de São Paulo) confirmed this decrease trend, breaking down data on health spending with individual litigation by month between 2015 and 2017.[70] Although these data have not yet been scientifically probed, these numbers are in line with the decrease observed in previous years (2012 to 2015) discussed by the works of Silva and Toma and colleagues, already mentioned in this article.
Furthermore, in June 2017, the CNJ in cooperation with the Ministry of Health launched an online consultation platform called e-NATJUS, which gathers technical information on all health technologies available in the Brazilian public health system.[71] This database, with technical notes, scientific analysis, and recommendations issued by advisory health committees in the country (NATs) and CONITEC, is easily accessible and aims at subsidizing judges in taking decisions on health care-related cases.[72]
Therefore, the establishment of technical committees and conciliation chambers all over Brazil aiming at dealing with and overcoming the problem of individual litigation for health care can be understood as policy responses which improved participation and capacity, advancing new forms of health care governance. These initiatives are in effect considered intergovernmental networks characterized by shared values and quality interactions, whose application to the field of health has the advantage of contributing to efficiency in the health system due to increased communication between the actors involved, identification of shortcomings in health demands flow, and the gathering of data for policy discussion purposes.[73]
Although more scientific evidence is needed, there are signs, as demonstrated in this article, that the new forms of collaborative governance between state actors in Brazil have the potential to prevent health care-related litigation. Moreover, conciliation and mediation point toward a new movement of removing courts from health care decision-making and thus creating a path for “de-judicialization” of health policies.[74]
Conclusion
The steady growth in the number of right to health claims in Brazil, which reportedly peaked in 2011 with more than 200,000 claims, led the judiciary to meet with institutions from other branches of the government.[75] This growth resulted in the Brazilian Supreme Court calling a landmark initiative, the Public Hearing on Health, in 2009. Since that public hearing, important changes have taken place at the judiciary level, such as the creation by CNJ of a working group focusing on health. These changes also developed at different levels of the executive branch of the government, affecting the HTA process and health care governance in the country. In this regard, the creation of CONITEC in 2012 is perhaps the highlight, establishing at least in principle a more transparent, participatory, and accountable HTA decision-making process in Brazil and having, therefore, the potential to contribute to the achievement of a more efficient and fair health system, not only through a more accountable allocation of health resources, but also through the availability of drugs to the population at large and not only to individual claimants.
The dialogical approach of the judiciary in this context opened the possibility of increased collaboration and partnerships between different state actors, such as state courts, state attorneys’ offices, public defenders’ offices, prosecutors’ offices, NATs and the CRLS, with the aim of reducing or better responding to individual health care litigation. Altogether, these institutional changes resulted in new forms of health care governance which are likely to improve participation and policy capacity through inter-institutional dialogue and the use of health professionals’ expertise.
Data available so far is very limited and is produced by the public institutions which run these initiatives; therefore it needs to be taken with caution. Yet states’ reports of a decrease in the number of lawsuits and spending on litigation in the last years are not implausible. If these trends are confirmed and consolidated, the paradox speculated by Wang may well become reality: by creating unfairness and inefficiency, individual litigation will have forced the Brazilian health system to become fairer and more efficient.[76]
Danielle da Costa Leite Borges, LL.M., MSc, PhD, is Postdoctoral Research Fellow at Scuola Superiore Sant’Anna, Pisa, Italy.
Please address correspondence to Danielle Borges. E-mail: danielle.borges@eui.eu
Competing interests: None declared.
Copyright © 2018 Borges. This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
All Portuguese-language material has been translated from Portuguese into English by the author.
[1]. S. Gloppen, “Legal enforcement of social rights: enabling conditions and impact assessment” Erasmus Law Review 2/4 (2009), pp. 465, 474.
[2]. D. Landau, “The reality of social rights enforcement.” Harvard International Law Journal 53 (2012), pp. 402, 413.
[3]. Gloppen (2009, see note 1).
[4]. D. W. L. Wang, Courts as Healthcare Policy-Makers: The Problem, The Responses to the Problem and Problems in the Responses, Direito GV Research Paper 75 Direito GV Research Paper Series – Legal Studies (São Paulo: Direito GV, 2013). Available at http://bibliotecadigital.fgv.br/dspace/bitstream/handle/10438/11198/RPS_75_final.pdf?sequence=1&isAllowed=y; F. F. Hoffmann and F. R. N. M. Bentes, “Accountability for social and economic rights in Brazil” in V. Gauri and D.M. Brinks (eds), Courting Social Justice (New York: Cambridge University Press, 2008), pp. 100-145; and O. L. M. Ferraz, “Are collective suits harder to enforce?” in M. Langford, C. Rodriguez-Garavito, and J. Rossi (eds), Social rights judgements and the politics of compliance: Making it stick (Cambridge, UK: Cambridge University Press, 2017), pp. 177-200.
[5]. See, for instance, Defensoria Pública do Estado do Rio de Janeiro, Deliberação CS/DPGE n° 124, from 20.12.2017, available at http://www.defensoria.rj.def.br/legislacao/detalhes/5485-DELIBERACAO-CS-DPGE-N%C2%BA-124-DE-20-DE-DEZEMBRO-DE-2017, and Conselho Superior da Defensoria Publica da União, Resolução, n° 134, from 07.12.2016, available at http://www.dpu.def.br/conselho-superior/resolucoes/37083-resolucao-n-134-de-07-de-dezembro-de-2016-fixa-o-valor-de-presuncao-de-necessidade-economica-para-fim-de-assistencia-juridica-integral-e-gratuita; See O. L. M. Ferraz, “The right to health in the courts of Brazil: Worsening health inequities?” Health and Human Rights Journal 11/2 (2009), pp. 33-45, and L. Reveiz; E. Chapman, R. Torres et al, “Right-to-health litigation in three Latin American countries: a systematic literature review,” Revista Panamericana de Salud Publica 33/3 (2013), pp. 213-222.
[6]. Ferraz.(see note 5).
[7]. M. M. Prado, “The debatable role of courts in Brazil’s healthcare system: Does litigation harm or help?” Journal of Law Medicine and Ethics 41/1(2013), pp. 124-137.
[8]. F. F. Hoffmann and F. R. N. M. Bentes (2008, see note 5).
[9]. D. L. Wang and O. M. Ferraz, “Reaching out to the needy? Access to justice and public attorneys’ role in right to health litigation in the city of São Paulo” SUR 18 (2013), pp. 159-179.
[10]. Federal Law nº 12.880 of 12 November 2013.
[11]. J. A. D. Oliveira, P.A.C. Fontes, “What Are They Complaining About After All? Study of Lawsuits Against a Health Plan Provider”, Revista de Direito Sanitário” 13/3 (2013), pp. 33-58.
[12]. O. L. M. Ferraz, “Harming the Poor through Social Rights Litigation”, Texas Law Review 89 (2011), pp. 1643-1668; and Wang and Ferraz (2013, see note 9).
[13]. J. Biehl, M. P. Sochal and J. J. Amon, “The Judicialization of Health and the Quest for State Accountability: Evidence from 1,262 Lawsuits for Access to Medicines in Southern Brazil” Health Hum Rights 18/1 (2016), pp. 209-220.
[14]. D. C. L. Borges, “Individual inputs and collective outputs: understanding the structural effects of individual litigation on healthcare in Brazil” in M. Hesselman, A. Hallo de Wolf and B. Toebes (eds), Socio-Economic Human Rights for Essential Public Services Provision (Oxon, New York: Routledge, 2017), pp. 241-255.
[15]. Federal Law n° 9.313 of 13 November 1996.
[16]. G. C. Chaves, M. F. Vieira and R. Reis, “Access to Medicines and Intellectual Property in Brazil: Reflections and Strategies of Civil Society”, Sur Revista Internacional de Direitos Humanos 5/8, (2008), pp. 170-198.
[17]. M. Ventura, L. Simas, V. L. Edais Pepe, R. Schramm, “Judicialization of the right to health, access to justice and the effectiveness of the right to health” Revista de Saúde Coletiva 20/1 (2010), pp. 77-100; and Wang (2013, see note 4).
[18]. M. Scheffer, A. L. Salazar and K. B. Grou, “O Remédio Via Justiça: um Estudo Sobre o Acesso a Novos Medicamentos e Exames em HIV/Aids no Brasil por Meio de Ações Judiciais’ (Brasília: Ministério da Saúde – Secretaria de Vigilância em Saúde – Programa Nacional de DST e Aids, 2005), available at http://bvsms.saude.gov.br/bvs/publicacoes/medic_justica01.pdf; and A. A. Miranda, “Aids e cidadania: avanços e desafios na efetivação do direito à saúde de soropositivos” in Direitos Humanos e HIV/Aids: avanços e perspectivas para o enfrentamento da epidemia no Brasil (Brasília: Ministério da Saúde, 2008), pp. 9-24, available at http://www.progepe.ufpr.br/caiss/documentos/direitos_humanos_e_aids.pdf.
[19]. A. M. Messeder, C. G. S. Osorio-de-Castro, V. L. Luiza, “Can court injunctions guarantee access to medicines in the public sector? The experience in the State of Rio de Janeiro, Brazil” Reports in Public Health 21/2 (2005), pp. 525-534.
[20]. L. Laurindo-Teodorescu and P.R. Teixeira, Histórias da aids no Brasil: as respostas governamentais à epidemia de aids, vols. 1 and 2 (Brasília: Ministério da Saúde/Secretaria de Vigilância em Saúde/Departamento de DST, Aids e Hepatites Virais, 2015).
[21]. A. S. Nunn, E. M. da Fonseca, and S. Gruskin, “AIDS Treatment In Brazil: Impacts and Challenges,” Health Affairs (Millwood) 28/4 (2009), pp. 1103–1113.
[22]. Hoffman and Bentes (2008, see note 5), p.126.
[23]. Wang (2013, see note 4).
[24]. J. D. F. Corvino, A crise do Sistema Único de Saúde e o fenômeno da judicialização da saúde. (Rio de Janeiro: Gramma, 2018).
[25]. Ministério da Saúde; Organização Pan-Americana de Saúde “Financiamento Público de Saúde” ECOS Series 1/1 (2013), http://bvsms.saude.gov.br/bvs/publicacoes/financiamento_publico_saude_eixo_1.pdf; S.F. Silva “The organization of regional and integrated healthcare delivery systems: challenges facing Brazil’s Unified Health System”, Ciência e Saúde Coletiva 16/6 (2011), pp. 2743-2762; C. D. B. S. Pinto and C. G. S. Osorio-de-Castro, “Pharmaceutical Services and litigation in small municipalities: a study in Mato Grosso do Sul”, Saúde em Debate 39 (2015), pp. 171-183; D. F. Macedo, J. A. R. Ataide, A. C. S. Costa et al., “Analysis of the Judicialization the right to health, Underfunding of the sector and Public Policy: A Case Study in the State of Alagoas”, Revista de Administração de Roraima-UFRR, 5/2 (2015), pp. 300-325.
[26]. Tribunal de Contas da União, Relatório Sistêmico de Fiscalização (Brasília, 2014), p. 122, available at http://portal.tcu.gov.br/biblioteca-digital/relatorio-sistemico-de-fiscalizacao-saude-1.htm.
[27]. Conselho Nacional de Justiça, Relatório de demandas relacionadas à saúde nos tribunais – dados enviados até junho de 2014, available at http://www.cnj.jus.br/images/programas/forumdasaude/demandasnostribunais.forumSaude.pdf.
[28]. D.W.L. Wang “Right to Health Litigation in Brazil: The Problem and the Institutional Responses”, Human Rights Law Review 15 (2015), pp. 617-641; L.M. Ribeiro, I.A. Hartmann “Judicialization of the Right to Health and Institutional Changes in Brazil”, Journal of Constitutional Research 3/3 (2016), pp. 35-52; V. G. Duarte, Arranjos e diálogos institucionais para enfrentamento da judicialização da saúde: uma analise dos modelos de assessoramento técnico (NAT’s), Master dissertation (Limeira, SP: Universidade Estadual de Campinas – UNICAMP, 2017) http://repositorio.unicamp.br/bitstream/REPOSIP/330565/1/Duarte_VanessaGenicia_M.pdf.
[29]. Documents on the public hearing. Available at http://www.stf.jus.br/portal/cms/verTexto.asp?servico=processoAudienciapublicaSaude.
[30]. N. Luhmann, Law as a social system (New York: Oxford University Press, 2004); A.O. Santos, M.C Delduque, A.V.M. Mendonça “Discourses in the Health Public Hearing and their impact on the decisions of the Supreme Court: an analysis to the theory of social systems” Saúde e Sociedade 24/1 (2015), pp. 180-188 (p. 188).
[31]. Ibid.
[32]. A. Meuwese and M. Snel, “‘Constitutional Dialogue’: An Overview”, Utrecht Law Review 9/2 (2013), pp. 123-140.
[33]. C. A. R. Garavito “El Activismo Dialógico y el Impacto de los Fallos Sobre Derechos Sociales” in R. Gargarella, Por una Justicia Dialógica: el Poder Judicial Como Promotor de la Liberación Democrática (Buenos Aires: Siglo Veintiuno Editores, 2014) pp. 211-244.
[34]. Portaria CNJ n° 650 from 20 November 2009. Available at www.cnj.jus.br///images/atos_normativos/portaria/portaria_650_20112009_18102012194714.pdf
[35]. Recomendação CNJ n° 31 from 30 March 2010. Available at http://www.cnj.jus.br///images/atos_normativos/recomendacao/recomendacao_31_30032010_22102012173049.pdf
[36]. Resolução CNJ n° 107 from 6 April 2010. Available at http://www.cnj.jus.br///images/atos_normativos/resolucao/resolucao_107_06042010_11102012191858.pdf
[37]. Documents on the public hearing from 11 December 2017. Available at http://www.cnj.jus.br/eventos-campanhas/evento/486-audiencia-publica-sobre-prestacao-da-jurisdicao-em-processos-relativos-a-saude.
[38]. World Health Organization, “Medical Devices”, Available at http://www.who.int/medical_devices/assessment/en/.
[39]. World Health Organization, Health Technology Assessment of Medical Devices, WHO Medical Devices Series (Geneva: WHO Press, 2011). Available at http://apps.who.int/iris/bitstream/handle/10665/44564/9789241501361_eng.pdf?sequence=1&isAllowed=y.
[40]. R. Kuchenbecker and C. A. Polanczyk, “Institutionalizing Health Technology Assessment in Brazil: Challenges Ahead,” Value in Health Regional Issues 1 (2012), pp. 257-261.
[41]. M. Bosi Ferraz, P.C. Soarez, P. Zucchi “Health technology assessment in Brazil: What do healthcare system players think about it?” São Paulo Medical Journal 129/4 (2011), pp. 198-205.
[42]. F. Lessa and M. Bosi Ferraz, “Health technology assessment: the process in Brazil.” Revista Panamericana de Salud Publica 41 (2017), p. e25.
[43]. Draft Bill from the Brazilian Senate PLS n° 338/ 2007 and Draft Bill from the Brazilian Senate PLS n° 219/2007.
[44]. M. Bellanger, P. Picon and L.T. Stuwe, “A Perspective on Health Technology Assessment Activities in Brazil”, ISPOR Latin America Consortium Newsletter, 3/4 (2015), pp. 1-3.
[45]. Ministério da Saúde. Relação Nacional de Medicamentos Essenciais -RENAME (Brasília, 2017). Available at http://bvsms.saude.gov.br/bvs/publicacoes/relacao_nacional_medicamentos_rename_2017.pdf.
[46]. http://conitec.gov.br/decisoes-sobre-incorporacoes
[47]. F. T. F. Elias and D. V. Araujo, “How health economic evaluation (HEE) contributes to decision-making in public health care: the case of Brazil”, Z. Evid. Fortbild. Qual. Gesundh. wesen (ZEFQ), 108 (2014), pp. 405-412.
[48]. Ministério da Saúde, Comissão Nacional de Incorporação de Tecnologias no SUS. Balanço 2012-2014 (Brasília, 2014) Available at http://conitec.gov.br/images/Artigos_Publicacoes/BalancoCONITEC.pdf.
[49]. I. R. Zimmermann, E. F. Oliveira, A. T. Vidal et al., “Evidence quality and recommendations on medicine coverage in the Brazilian Public Health System: a retrospective analysis”, Revista Eletrônica de Gestão & Saúde 6/4 (2015), pp. 3043-3065.
[50]. R. Caetano, R. M. da Silva, E. Militão Pedro, I. A. G. de Oliveira, et al. “Incorporation of new medicines by the National Commission for Incorporation of Technologies”, Ciência e Saúde Coletiva, 22/8 (2017), pp. 2513-2525.
[51]. Comissão Nacional de Incorporação de Tecnologias no SUS, Mudanças no Canal de Comunicação com o Judiciário. Available at http://conitec.gov.br/ultimas-noticias-3/mudancas-no-canal-de-comunicacao-com-o-judiciario.
[52]. E.P. Silva, “O papel dos comitês técnicos de especialistas na gestão de novas tecnologias em saúde”, in Tereza Setsuko Toma et al. (eds), Avaliação de Tecnologias de Saúde: desafios e propostas para a gestão (São Paulo: Instituto de Saúde, 2015), pp. 57-63, available at http://www.saude.sp.gov.br/resources/instituto-de-saude/homepage/temas-saude-coletiva/pdfs/ats_inova_saude_capa_miolo.pdf.
[53]. T.T. Toma, A.C. Soares, P.S.F. Siqueira, R. Domingues “Strategies to deal with drug lawsuits in the state of São Paulo, Brazil”, Cadernos Ibero-Americanos de Direito Sanitário 6/1 (2017), pp. 35-54.
[54]. F. S. Vieira, Evolução do gasto com medicamentos do Sistema Único de Saúde no período de 2010 a 2016 (Brasília: Instituto de Pesquisa Econômica Aplicada -Ipea, 2018), available at http://www.ipea.gov.br/portal/images/stories/PDFs/TDs/180117_td_2356.pdf.
[55]. Ministério da Saúde, Comissão Nacional de Incorporação de Tecnologias no SUS. (2014, see note 48).
[56]. World Health Organization. Health systems governance. Available at http://www.who.int/healthsystems/topics/stewardship/en/
[57]. S. L. Greer; M. Wismar; J. Figueras and C. Mackee, “Governance: a framework” in S. L. Greer; M. Wismar; J. Figueras Strengthening Health System Governance: better policies, stronger performance. (European Observatory on Health Systems and Policies Series, 2016).
[58]. Ato Normativo 5/2012 from 31 January 2012. Available at http://www4.tjrj.jus.br/biblioteca/index.asp?codigo_sophia=152866&integra=1.
[59]. K. R. T. R. de Castro, Os juízes diante da judicialização da saúde: o NAT como instrumento de aperfeiçoamento das decisões judiciais na área da saúde. Master dissertation (Rio de Janeiro, RJ: Fundação Getulio Vargas – FGV Direito Rio, 2012), pp. 61 and 64. Available at http://bibliotecadigital.fgv.br/dspace/bitstream/handle/10438/9769/K%C3%A1tia%20Regina%20Tinoco%20Ribeiro%20de%20Castro.pdf?sequence=1&isAllowed=y.
[60]. Law n. 13.105 from 16 March 2015. Available at http://www.planalto.gov.br/ccivil_03/_ato2015-2018/2015/lei/l13105.htm.
[61]. Recomendação CNJ n° 31 (see note 35); Duarte (2017, see note 28), p.66.
[62]. T. Cieglinski, “Justiça gaúcha reduz gastos com saúde,” Agência CNJ de Noticias (April 19, 2017) Available at http://www.cnj.jus.br/noticias/cnj/84643-justica-gaucha-reduz-17-os-gastos-com-judicializacao-da-saude.
[63]. Silva (2015, see note 52).
[64]. Procuradoria do Estado do Rio de Janeiro. Câmara de Resolução de Litígios de Saúde tem Resultado Positivo. Available at http://www.rj.gov.br/web/pge/exibeconteudo?article-id=1886157.
[65]. R.C.M. Guimarães, P.H. Di mais Palheiro, Medidas adotadas para enfrentar a judicialização na Secretaria de Saúde do Estado do Rio de Janeiro e a experiência da Câmara de Resolução de Litígios de Saúde (Conselho Nacional de Secretários de Saúde, CONASS, 2015) Available at http://www.conass.org.br/biblioteca/pdf/colecao2015/CONASS-DIREITO_A_SAUDE-ART_33.pdf.
[66]. Procuradoria do Estado do Rio de Janeiro. Câmara de Resolução de Litígios de Saúde, da PGE-RJ, evita mais de 15 mil processos na Justiça. Available at https://www.pge.rj.gov.br/mais-consenso/camara-de-resolucao-de-litigios-de-saude-crls
[67]. P. Paim, A. Marqueto and I. O. Lopes, “Câmara Permanente Distrital de Mediação em Saúde: experiência do Distrito Federal”, Revista do Conselho Nacional de Secretários de Saúde – CONASS (2015). Available at http://www.conass.org.br/biblioteca/pdf/colecao2015/CONASS-DIREITO_A_SAUDE-ART_17B.pdf.
[68]. Conselho Nacional de Justiça. Câmara de Conciliação da Saúde resolve 80% dos casos na Bahia (30 August 2017). Available at http://www.cnj.jus.br/noticias/judiciario/85328-camara-de-conciliacao-de-saude-resolve-80-dos-casos-na-bahia.
[69]. G. Cabral, “Pedidos de remédio na Justiça caem, e SP evita gastos de R$ 205 milhões”, Folha de São Paulo (March 10, 2018). Available at https://www1.folha.uol.com.br/cotidiano/2018/03/pedidos-de-remedio-na-justica-caem-e-sp-evita-gastos-de-r-205-milhoes.shtml.
[70]. Secretaria de Estado da Saúde do Governo de São Paulo. Available at http://www.cnj.jus.br/files/conteudo/arquivo/2018/01/b3a6bc2722f4f3c5977a399a686ef3af.pdf.
[71]. Conselho Nacional de Justiça, e-NatJus, available at www.cnj.jus.br/programas-e-acoes/forum-da-saude/e-natjus .
[72]. Consellho Nacional de Justiça. Suporte para decisões sobre saúde começa pelo Paraná (3 July 2017). Available at http://www.cnj.jus.br/noticias/cnj/85032-sistema-de-suporte-a-decisoes-em-saude-comeca-a-ser-usado-no-parana.
[73]. A. M. de Souza, A atuação em rede de instituições governamentais na resolução de conflitos sobre demandas sanitárias no Rio de Janeiro, Master Dissertation (Rio de Janeiro, RJ: Fundação Getulio Vargas – FGV, 2016). Available at http://bibliotecadigital.fgv.br/dspace/bitstream/handle/10438/16052/Disserta%C3%A7%C3%A3o_ver_final.pdf?sequence=1&isAllowed=y.
[74]. A. Barbosa and G. Schulman, “The de-judicialization of health: mediation and interinstitutional dialogues”, Revista Bioética 25/2 (2017), pp. 290-300.
[75]. Conselho Nacional de Justiça, Relatório de demandas relacionadas à saúde nos tribunais – dados enviados até 2011, available at http://www.cnj.jus.br/images/programas/forumdasaude/relatorio_atualizado_da_resolucao107.pdf.
[76]. Wang (2015, see note 23), p. 641.
