Volume 21/1, June 2019, pp 63 – 79
Alyssa Mari Thurston, Federico Andrade-Rivas, and Jerry M. Spiegel
Abstract
Researchers investigating breast milk contamination face substantive ethical dilemmas regarding how biomonitoring results should be conveyed, with limited guidance available to help them. To identify effective processes for undertaking such research, we sought to critically assess practices being followed in reporting results. To consider how researchers have reported on this and related ethical issues, we searched three English-language databases for articles published between 2010–2016 on measuring presence of pesticides in breast milk. Data on report-back processes and discussed ethical issues were charted from retained articles (n=102). To deepen our understanding of issues, we further consulted authors (n=20) of retained articles through an online survey. Quantitative data from surveys were tabulated and qualitative data were analyzed thematically. Of 102 articles, only two mentioned sharing results with subjects, while 10 out of 20 survey participants confirmed that they had indeed conducted report-back in their studies. Articles discussing ethical considerations were few (n=5), although researchers demonstrated awareness of common ethical debates to inform report-back decisions. Our review suggests that greater explicit attention should be given to practices of engaging study subjects and their communities in contamination studies so that an evidence base on best ethical practices can be more readily available.
Introduction
Pollution, including chemical pollution, not only undermines the fundamental right to a safe and healthy environment, but has been identified by global disease burden assessments as one of the greatest threats to human health.1 Responding to this, human biomonitoring (HBM) is increasingly pursued as a way to assess chemical exposure and provide additional evidence to that obtained through environmental monitoring of soil, water, and food.2 Analysis of biomarkers of exposure in samples of blood, urine, hair, nails, breast milk, and saliva directly assess the body burden of hundreds of chemicals and their metabolites.3 While HBM provides a way to assess chemical exposure at individual and population levels, an emergent issue within the field of toxicology and environmental health is the ethical challenge of results disclosure and communication with research subjects in studies using HBM as a tool for environmental health risk assessment. The last decades have seen a rise in debates among groups of concerned scientists, ethicists, activists, and other stakeholders on how appropriate it is to communicate biomonitoring results to research subjects, what information should be communicated, and how communication should be conducted.4
In recognition of these challenges, we sought to draw on identified best ethical practices to inform the conduct of our research responding to an Ecuadorian community’s concerns about the impact of intensive pesticide use, as part of an ongoing international research program investigating associations between food systems and health equity. The ethical issues were of particular importance to us as our research applies the Latin American Social Medicine orientation to health equity, which considers those affected as not only participants in the research process but also as active agents (recognized as subjects in the language of this approach) in the process of pursuing their right to health.5 This is in a manner consistent with the participatory action research orientation to community engagement in “Western” health research approaches that has challenged the more passive framing of “research subjects” as essentially an entity for researchers to observe.6
With our study approach including measurement of pesticide concentrations in breast milk, we were especially apprehensive that reporting results in conformity with the right to know about the health threat posed by exposure to toxins could cause undue fear among mothers and discourage breastfeeding, which itself has been recognized as a human right of the mother/child dyad.7 We quickly observed that despite the growing body of literature highlighting issues involved and the benefits of potential approaches, no assessment of results communication practice, strategies, and considerations had, to our knowledge, been conducted to inform this research. To address this knowledge gap, we initiated this sub-study to critically assess how results communication in breast milk contamination studies are conducted and reported.
Conceptualizing the challenges
To situate our study objectives within existing discussions on the issue, here we aim to synthesize key debates, broadly categorized into 1) whether or not to communicate biomonitoring results to research subjects; and 2) if communication is deemed appropriate, who should communicate results with subjects, what should be communicated, and how should it occur.
To communicate or not
Decisions to conduct report-back are influenced by a variety of ethical considerations, including scientific uncertainties associated with biomonitoring data and their insights into potential health effects—as well as what can be done with this information. While HBM is undoubtedly useful to provide evidence that exposure and uptake of a pollutant in question has taken place, results can only provide a snapshot of an individual’s exposure to a particular chemical and cannot reflect exposure throughout one’s lifetime, the interaction of the chemical with other body burdens, or potential sources of the exposure.8 Furthermore, the considerable lag in scientists’ ability to understand individual health implications of exposures in comparison to the rapid advancement in technology to detect pollutants challenges scientists to find meaning in single-measurement data and ways to appropriately advise subjects on personal exposures.9 Uncertainties with the value and implication of HBM data lead some to argue that report-back should only occur if results have known association to an adverse health outcome to avoid causing unwarranted fear and anxiety over results that may have no clinical relevance.10 Some researchers measuring exposure of pollutants with established clinical levels (for example, lead or mercury) have conducted timely report-back to subjects whose results exceed acceptable levels.11
The concern of causing undue fear is underscored in sensitive cases like biomonitoring of breast milk, as some emphasize that anxiety over individual-level results may cause mothers to reduce or stop breastfeeding altogether.12 The widespread consensus that breast milk is the most appropriate form of nutrition for infants thus makes the risk of HBM results influencing mothers’ decisions regarding breastfeeding a particularly difficult ethical challenge. However, evidence on actual behavioral and psychological impacts of body burden knowledge on subjects remains inconclusive. Findings from a limited number of studies on this issue are mixed, where some have found that subjects experience some degree of anxiety, frustration, or guilt over their results, while others found subjects are not excessively worried about their individual results or can even feel empowered to take action to reduce their exposures.13 A survey by Geraghty et al. based on hypothetical scenarios suggested that concerns over breast milk contamination may cause mothers to terminate breastfeeding prematurely, while Wu et al. found that mothers who received individual breast milk biomonitoring results in their study did not change their breastfeeding behavior.14
Another source of debate stems from ethical considerations of HBM report-back for marginalized, disadvantaged, and vulnerable communities or cultural groups that may be at heightened risk of exposure to harmful toxicants based on historical and existing environmental injustices.15 Some express concerns on communication of results in this context as potentially further marginalizing vulnerable subjects or undermining the gravity of underlying political, historical, and social issues by employing a primarily individualized risk assessment lens to environmental health problems that manifest at a broader scale.16 In communicating results to socioeconomically disadvantaged subjects, researchers express concern that knowledge of body burdens among subjects with limited capacity and means to reduce exposures would only cause feelings of frustration and powerlessness.17 On the other hand, Adams et al. found that through open communication and involvement of trusted community organizations, researchers are able to inform subjects from disadvantaged backgrounds in ways that promote actions to mitigate exposures.18 Others view disclosing individual results as an important way to rectify historical abuses, exploitations, and neglect of individuals and communities involved in research by diminishing disparities in information access and autonomy, as well as by promoting transparency and building trust between researchers and subjects.19 The potential for scientific evidence on body burdens to demonstrate the injustices suffered by marginalized groups is also rationalized as a reason to communicate results to subjects, communities, and policy-makers, given its potential to spur action for environmental management.20
Alongside these debates, guidance and decisions on disclosure of HBM results are also varied among Institutional Review Boards (IRBs) that oversee the ethical pursuit of research. Some IRBs have denied researchers’ requests to communicate results with subjects due to similar concerns of causing undue fear among subjects and uncertainty in the value and meaning of biomonitoring data, while some have supported report-back if these scientific uncertainties were clearly explained to subjects.21 In other instances, IRBs have fully supported report-back of results or were inconsistent in their decisions.22 Variation in IRBs decisions and rationale to approve or reject report-back highlights the lack of consensus on appropriate practices. A study by Ohayon et al. suggested that IRB members with limited experience with HBM were more concerned with scientific uncertainties and potential harms of results communication, whereas those with more experience viewed report-back favorably and as the moral course of action.23
Within the multitude of debates that question the ethics and suitability of results disclosure, proponents of report-back firmly point to researchers’ moral obligation to communicate results, subjects’ right to know their personal body burdens, and the benefits of subjects knowing their individual results.24 These benefits include improving environmental health literacy among subjects, encouraging individual and collective action to mitigate exposures, as well as enriching research itself by improving study participation and generating new perspectives.25 Furthermore, Quigley argues that denying subjects decision-making information to reduce exposures would be unjust if it turns out that worry was not undue and detected levels may indeed cause adverse health effects.26 Importantly, a growing number of studies indicate that subjects themselves overwhelmingly want to know their individual results.27 Despite some researchers’ desire to communicate HBM results to subjects, there is a general lack of guidance on appropriate report-back strategies and in particular, on sensitive cases like biomonitoring of breast milk or involvement of subjects from marginalized or disadvantaged backgrounds.28
Results communication strategies: who, what, and how
If report-back is deemed appropriate on the basis of these ethical considerations, researchers are faced with more debates and difficult decisions regarding communication strategies. In terms of who should communicate results to subjects, some argue scientists are best positioned for report-back, as they possess knowledge on the uncertainties surrounding their research and ability to ensure that no misinterpretation of the data occurs.29 Others suggest communication should occur by researchers in conjunction with, or entirely by, health professionals who are able to relay the clinical significance of subjects’ individual results, while some researchers express concern that clinicians may be limited in their ability to advise on individual results without specific knowledge and training on environmental health.30 For breast milk biomonitoring, involving lactation specialists or NGO workers with experience in breastfeeding promotion as part of the report-back strategy has been noted as good practice.31 Studies have also recommended involving counselors and local community representatives in the report-back process, as well as a contact person that subjects can refer to for inquiries on their results throughout the duration of the study.32 In cases where researchers are sharing control of the study through participatory research strategies, it may be considered that local collaborators lead communication and establish an approach to report-back adapted to community needs and context.33
In terms of what to communicate, several guidance documents call for report-back of individual-level results.34 Some researchers recommend reporting individual results along with the study aggregate results, or results from comparable studies, in order to contextualize and promote understanding of personal levels of contamination.35 Where individual implications of exposures and specific health outcomes are unknown, some suggest report-back of aggregate study results instead of individual results.36 Moreover, aggregate results may also be most appropriate in cases where individualized results may cause discrimination of individuals (for example, in obtaining employment or insurance).37 Beyond the type of results to be included for report-back, researchers suggest including explanations on what is known on health implications and exposure mitigation as part of what is communicated to subjects.38
In addition, researchers have offered suggestions with regard to strategies on how to communicate biomonitoring results to subjects. Some researchers have reported results through reports and workshops or meetings, while the World Health Organization (WHO) has created an information sheet for dissemination to mothers involved in breast milk biomonitoring studies.39 Researchers emphasize the importance of offering results in a variety of ways, including text, graphs, diagrams, or pictures, to be mindful of different literacy levels and communication preferences.40 In terms of mode of report-back, results have been communicated in person to give subjects an opportunity to ask questions, while a review of participants’ preference found that results shared via mail were deemed satisfactory, but some preferred face-to-face contact in general or in cases of negative results.41 Both passive and active forms of report-back have been practiced, in which subjects could contact the research team if they wanted to receive their individual results or where the researcher actively offered subjects their results.
Methods
Acknowledging the lack of consensus regarding reporting back HBM results, and the particular ethical concerns of human breast milk pesticide contamination studies, we designed a methodology to assess how results communication is being discussed and conducted in this type of research.
Review of literature
To thoroughly map how results communication is being conducted and reported in breast milk contamination studies, we conducted a scoping review guided by the Arksey and O’Malley approach for scoping studies, as revised by Levac et al.43 We searched three prominent databases (PubMed, Medline, and Toxline) for peer-reviewed articles related to pesticide contamination of breast milk, using the keywords “pesticide”; “breast milk” or “human milk”; and “contamination.” The scope of the search was limited to articles published between January 2010 and October 2016, when our team started the review process. We excluded articles that were 1) not published in English; 2) unrelated to the topic or did not analyze human breast milk as part of the study methodology; 3) focused on the methodology of analyzing breast milk and not on exposure to a pollutant; and 4) reviews. Two reviewers were engaged in the review of articles for inclusion, with a third global health practitioner with expertise in environmental health adjudicating discrepancies.
Selected articles were then examined to assess whether or not they mentioned having conducted report-back to research subjects. If studies reported communicating results, we charted the chosen method of report-back (for example, a brochure or workshop) and the type of data that was reported, including individual-level, aggregate-level, or pooled results (that is, samples from study population mixed for analysis). Articles with any discussions on ethical considerations of report-back relating to breast milk contamination were also noted. As well, we recorded if articles investigated ‘exposure’ or ‘effect’, where the purpose of ‘exposure’ studies was to document exposure levels of some pollutant in breast milk and the purpose of ‘effect’ studies was to investigate the presence of some suspected health effect. Two reviewers independently extracted and charted data in terms of designated categories (Table 1).

Survey of researchers
Research teams who produced the articles included in our review were then contacted in order to obtain further insights with regards to ethical discussion and results communication beyond the information available in the assessed publications. Teams were requested to participate in a survey of ten quantitative and qualitative questions (see Appendix 1). Contact persons for each article were identified and duplicates of individuals who served as contacts for more than one retained study were removed. Email invitations to participate in the survey included preliminary findings from the review to engage participants with the critical issues identified and provide opportunities to address knowledge gaps. Survey results were collected anonymously to ensure participants were able to voice their opinions freely on the sensitive topic. Ethical approval was granted by the University of British Columbia’s Behavioural Research Ethics Board.
Results for closed-ended questions were tabulated, while results for open-ended questions were organized and analyzed by emergent themes. Two researchers conducted this process independently, and themes obtained were later discussed and reflected upon to produce a unified analysis.
Results
Study selection and characteristics
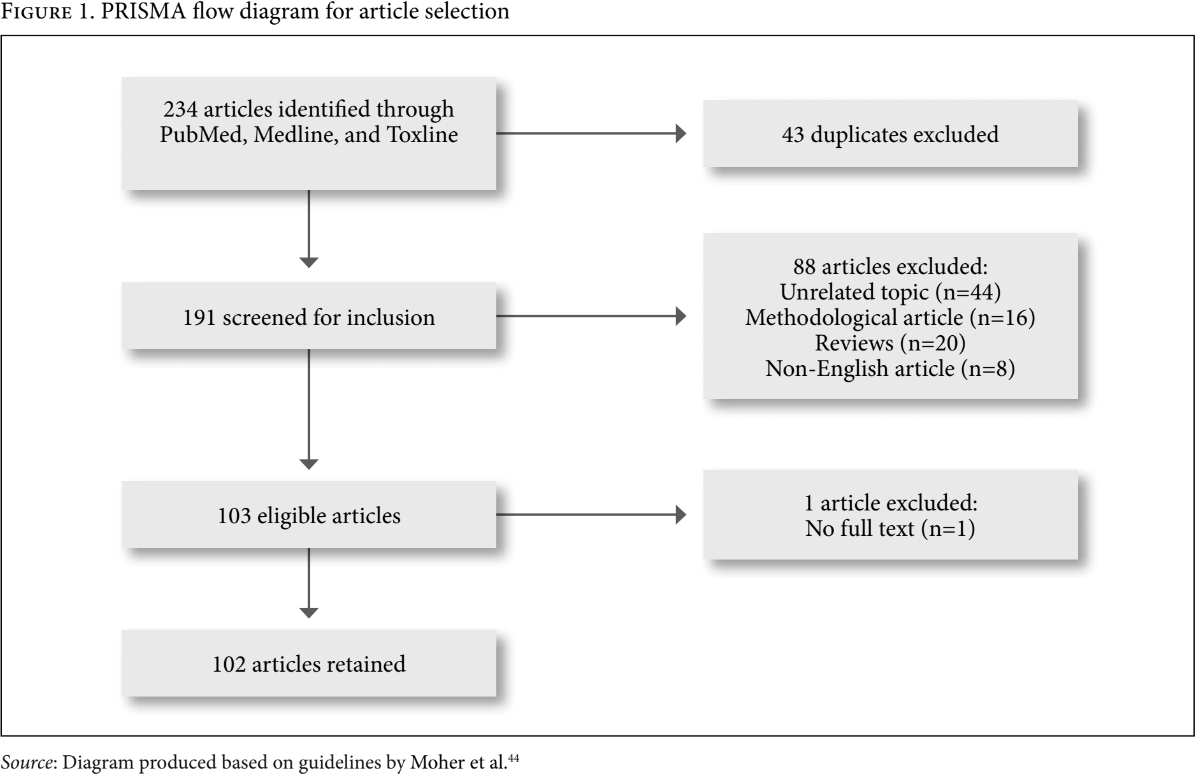
After removal of 43 duplicates, 191 articles were screened for inclusion based on our eligibility criteria. All articles retained were published in English and measured some level of exposure to a pollutant via breast milk biomonitoring. Articles on unrelated topics (n=44), articles focused on methodology of how to conduct breast milk biomonitoring (n=16), review articles (n=20), non-English (n=8), and articles without full text (n=1) were excluded from our search. This inclusion/exclusion strategy resulted in the retention of 102 articles from 234 articles that were identified (Figure 1).
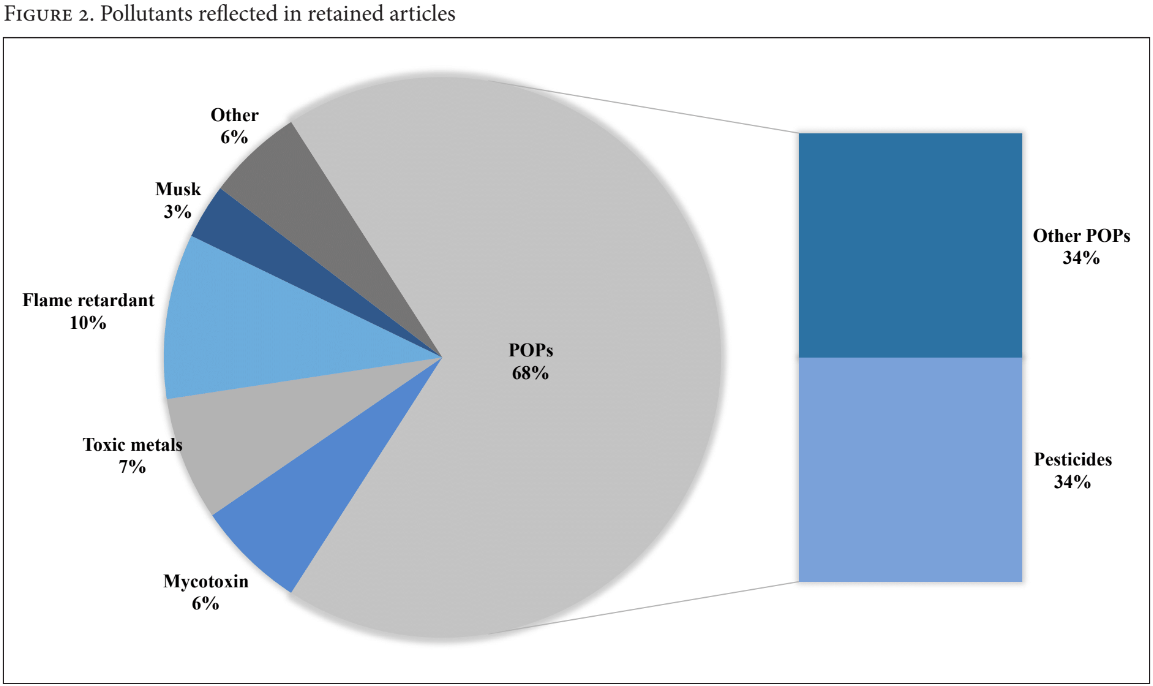
Of the 102 retained articles that measured exposure to some pollutant through breast milk biomonitoring, 14 articles also investigated some suspected health effect. While “pesticide” was used as a search term to focus the review on our interest in breast milk contamination of pesticides, we retained any article returned in the search that measured other pollutants, as similar ethical issues on report-back would prevail regardless of the type of pollutant. We made note of studies investigating exposure to mercury and lead, as pollutants with established guidance values may have impact on researchers’ decisions to communicate results with their subjects.45 Among all pollutants reflected in retained articles, Persistent Organic Pollutants (POPs) consisted 68%, where 35% of this number was specific to pesticides. Musk, flame retardants, toxic metals, mycotoxins, and other pollutants (for example, bacteria, radioactive pollutants, and mineral oils) were also reflected in articles (Figure 2).
Review of literature
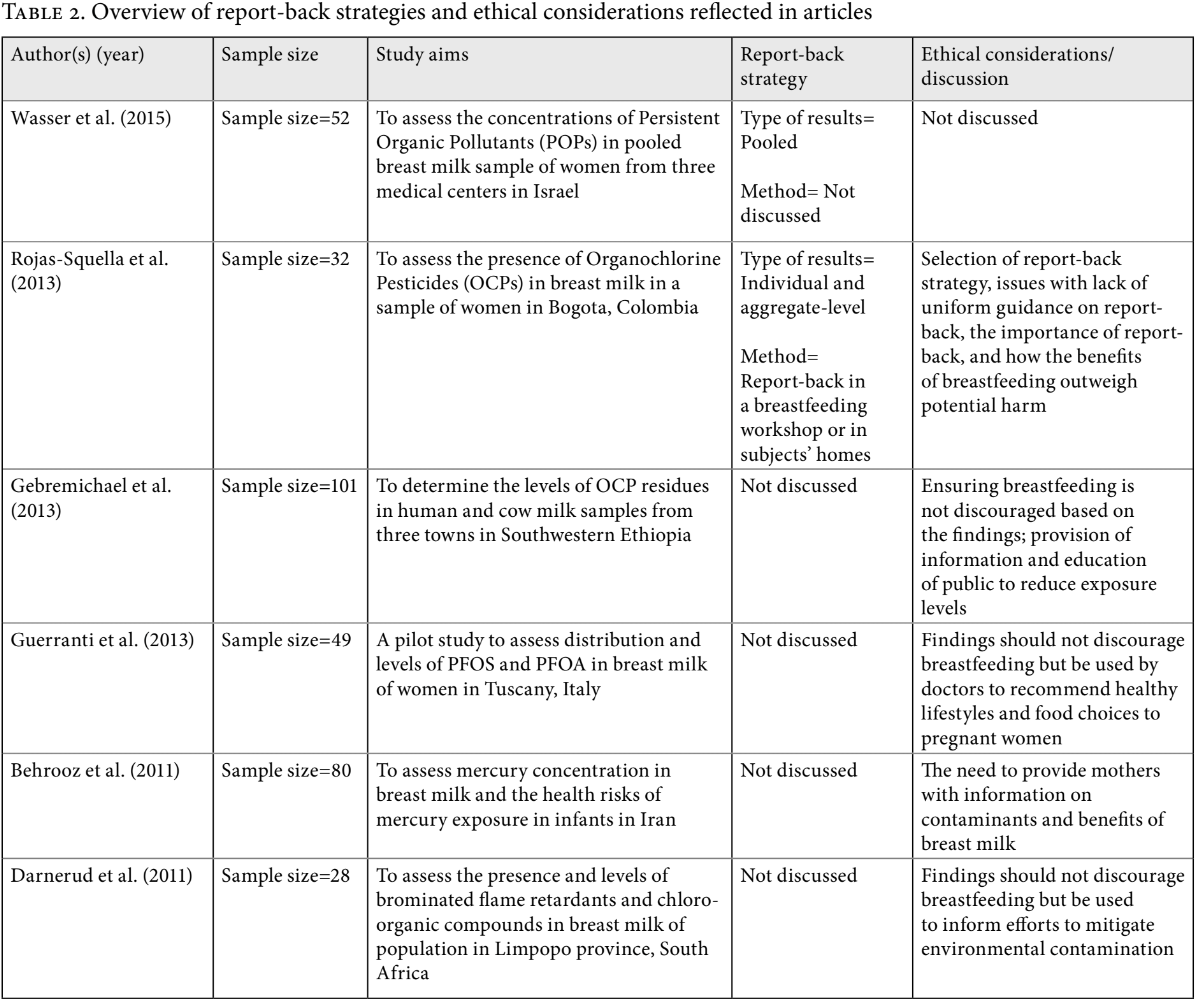
First, our team sought to determine how many articles reported communicating results to participants within their publications. Based on the review of articles, 100 out of 102 articles did not indicate any report-back of results to research subjects. Of the two articles that indicated communication of results to subjects, Rojas-Squella et al. reported individual and aggregate-level results through a breastfeeding workshop and in subjects’ homes, while Wasser et al. reported pooled results to subjects (see Table 2).46 Rojas-Squella et al. explained that finding uniform guidance on appropriate report-back methodology was a challenge, necessitating consultation with other researchers who had conducted similar studies in developing countries.47 Their decision to communicate both individual and aggregate-level results was based on recommendations from other researchers, as it was suggested that aggregate results contextualize and promote better understanding of individual results.48 Of the 12 articles that were labeled as ‘effect’ studies and the 11 articles investigating exposure to mercury and/or lead, none discussed conducting report-back.
Table 2 summarizes the report-back strategies and ethical considerations reported in the articles included in this study.49 Only five of the reviewed articles included some discussions on ethical considerations relating to report-back of breast milk biomonitoring results. These primarily focused on how findings of studies should not discourage breastfeeding. Rojas-Squella et al. explained that while the adverse health effects of their pollutant of interest are not entirely known, the benefits of breastfeeding likely outweigh potential harms.50 Others highlighted the importance of report-back and providing information to subjects, and stated that findings should be used to inform subjects’ choices to mitigate exposure and overall efforts for environmental management.51
Survey of research teams
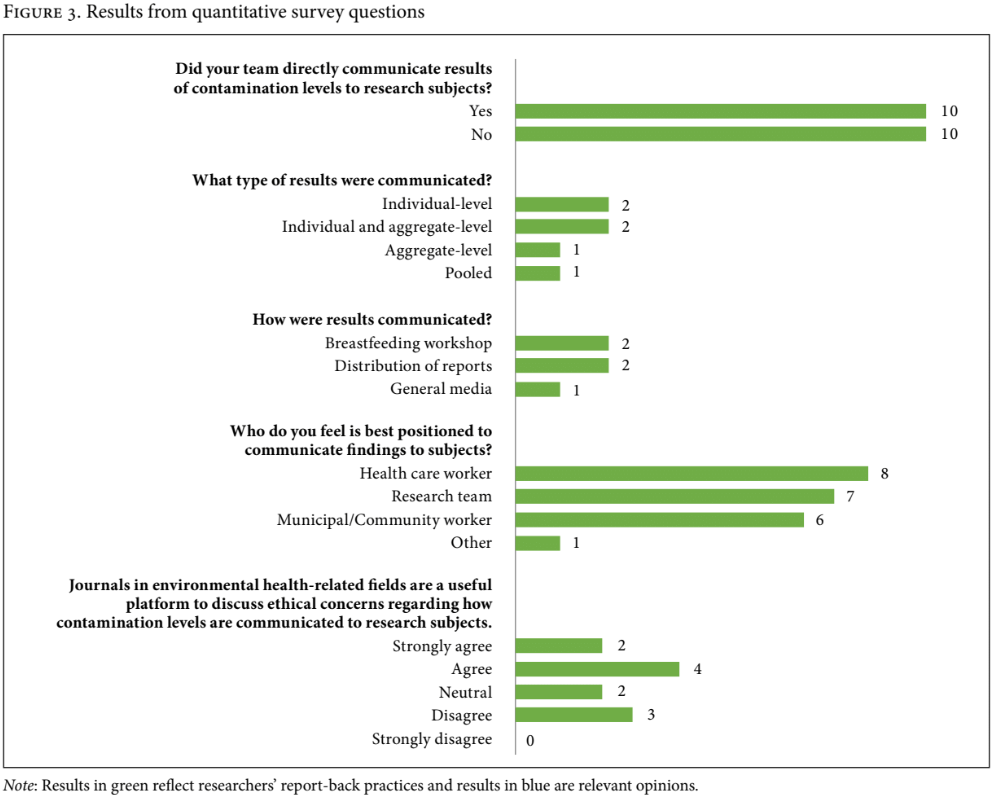
After removal of duplicate contact persons and invalid contact information, we contacted 82 research teams from retained articles and 20 participated in our survey (24.4% response rate). From quantitative survey questions, we found that 10 out of 20 researchers had conducted report-back in their studies, while the other 10 had not. It is worth noting that survey responses were collected anonymously, thus it is possible that the authors of the two articles identified to have conducted report-back through the literature review were also among the 10 identified in the survey. Researchers had communicated individual-level, aggregate-level, and pooled results to subjects through breastfeeding workshops, distribution of reports, and general media (see Figure 3). One respondent elaborated that their workshop included health care workers who were experts in the field of environmental exposure and breastfeeding promotion. Another indicated that while their team had distributed reports to subjects, they would have ideally included a workshop despite being difficult to organize. Researchers believed that health care workers, research teams, and municipal/community workers had similarly important roles in report-back processes (see Figure 3).
Researchers had mixed perspectives on the usefulness of academic journals in environmental health as platforms to discuss ethical considerations regarding report-back (see Figure 3). One researcher who agreed with pursuing this avenue elaborated that their team had previously used academic journals in this field to discuss experiences with report-back (but had not done so for the study included in this review, as they had used anonymized samples from biobanks).
From qualitative questions, three broad themes emerged on considerations behind report-back and related challenges. The first theme covered various perspectives and reasons for conducting report-back. Respondents discussed communicating results with subjects to ensure that they were provided the right message and that study findings did not discourage breastfeeding. Three respondents mentioned that report-back should be conducted to inform subjects on their exposures and the topic in general, while one added that report-back was a form of compensation to subjects for participating in their study. Others specified that report-back was only conducted in cases where results appeared to be of clinical significance or were only permitted to do so by IRBs (see Box 1).

The second theme that emerged focused on researchers’ rationales for not conducting report-back. Concern that communication of results might discourage breastfeeding was prominent in this regard. Additional attention was drawn to communities’ level of understanding, scientific uncertainty, and inabilities to mitigate exposure. One researcher further explained that they decided against report-back because the community’s level of understanding on the topic may have caused more harm in the context of their study. Explicit denial of report-back specified by IRBs was reported by two researchers, with one attributing this to concerns of causing undue fear, as well as issues with scientific uncertainty and subjects’ inability to mitigate exposures. Other reasons that were raised included report-back not being the objective of the study, and lack of contact with subjects due to logistical issues or use of biobank samples. However, some researchers who lacked contact with subjects explained that they believe report-back is important and must be done without causing anxiety among mothers (see Box 2).

The last emergent theme was on challenges that researchers identified regarding processes of reporting back on findings. A common issue was lack of guidance within publications and from IRBs, where one respondent explained having to contact other researchers directly as a result of difficulties with finding advice on report-back within publications. Navigating the duty to share results with subjects while ensuring results do not cause undue fear, as well as limitations of scientists’ understanding of health implications and mitigation of exposures, were raised as challenges to conducting appropriate report-back. One respondent discussed the risk of miscommunication due to researchers’ lack of training in risk communication as a challenge (see Box 3).

Discussion
While studies examining selected researchers’ perspectives on report-back through interviews or focus groups have suggested a level of awareness of ethical issues among scientists, this orientation was not widely reflected in our study of contaminant studies published between January 2010 and October 2016.52 With only 2 out of 102 articles reviewed for this study explicitly presenting experiences in report-back of results to subjects, it is apparent that the vast majority of research teams either did not communicate results with subjects, chose not to discuss strategies and considerations for report-back within their articles, or potentially chose to discuss this in other publications or platforms. Articles that included at least some discussion of the sensitive nature of report-back, the importance of encouraging breastfeeding despite findings of contamination, the need to communicate findings with subjects, or other relevant ethical topics were similarly few (5 out of 102).
We initially hypothesized that scientists conducting biomonitoring research may be motivated to share experiences and best practices for report-back within publications, partly in recognition of the lack of formal guidance and emergence of debates on related ethical issues over the years, as documented by LaKind et al. in their 2008 article on polybrominated diphenyl ethers in breast milk in the United States.53 To better understand research practices in reporting results, we consulted research teams that produced the articles in our scoping review to understand gaps between what researchers reported in publications and what they may have practiced in the field. While acknowledging the likelihood that researchers who chose to participate in our survey were biased toward greater awareness, interest, or experience with report-back, our findings indicate that report-back is seemingly being conducted to a greater degree in practice than what articles may suggest, and decisions to discuss practices of results communication remain limited.
Researchers’ decisions regarding report-back strongly reflected discussion of critical issues and debates in the literature. Key challenges identified included lack of guidance, navigating duty to report with associated potential of this to cause harm, scientific uncertainty, and inability to advise on mitigation of exposures.54 Researchers’ lack of training in risk communication as a challenge to report-back was a unique perspective raised in this survey and supports assertions that researchers need training in report-back techniques.55
Echoing one of the leading arguments against report-back, researchers who had not reported their results to subjects explained that their decision was guided by concerns with causing anxiety and discouraging breastfeeding as a result of mothers’ knowledge of their body burdens. Interestingly, some researchers used this same argument of not wanting to discourage breastfeeding to justify report-back in explaining that they had shared results precisely to ensure this would be not misinterpreted and the right message for promotion of breastfeeding was disseminated. While the potential of negatively influencing breastfeeding behavior is overwhelmingly used as a reason against report-back, this tendency highlights how without clear evidence and consensus on the process of reporting results to subjects being studied, ethical considerations can be construed and acted upon differently depending on researchers’ interpretations or stances on the issue.56 IRBs are in the position to give guidance and oversight on research ethics, but they also rely on researchers and research findings to provide guidance on relatively unknown subject matters like ethics in report-back for biomonitoring studies.57 Knowledge exchange on experiences among researchers and IRBs can indeed serve to mutually reinforce understanding of ethical dilemmas and best practices for report-back.
On the topic of not communicating findings to limit undue fear, one researcher in our survey further explained that their team was concerned with causing worry among subjects due to the community’s level of understanding on the subject. Concern over this underlying driver of undue fear is not as widely reflected in the literature, as studies increasingly point to subjects’ ability to understand and cope with topics in environmental contamination and individual biomonitoring results, regardless of socioeconomic background.58 However, it is possible that this type of perspective is more prevalent than is evident in the literature, as there are limited platforms for researchers to raise such concerns free of judgment. This points to an urgent need for guidance and sharing of experiences among researchers, as preconceptions of context and capacity of subjects can reaffirm neocolonial relationships between researchers and subjects and neglect subjects as beneficiaries of research. Another justification raised in our survey for not conducting report-back was that data was collected and analyzed “purely for research purpose,” thereby completely bypassing subjects from research benefits. The issue of who should be gaining from advancements in knowledge goes beyond the field of environmental health, as it points to the common oversight within global health research, whereby researchers are—or are perceived to be—the primary beneficiaries of data collected from research subjects and communities rather than as collaborators who work with communities in their pursuit of a right to health such as by contributing to community-led advocacy for the reduction of exposure to environmental toxins.59
Moreover, global health research conducted among marginalized populations should not only understand community engagement as a strategy to achieve a human rights mandate by improving health or reducing hazardous exposures but also consider community engagement as a rights-based process with the potential to strengthen communities beyond the boundaries of the study or intervention.60
In fact, consideration of the scale that is being examined in studies can provide some guidance as to appropriate ethical approaches that should be taken. While HBM studies of contamination record contamination at the scale of the individual, the study of environmental pollutants is experienced at a population level—at the scale of community or larger area. In this regard, documenting the degree of contamination associated with patterns of exposure in settings where this has intensified can serve to signal a need for modification. In line with the concerns registered by indigenous studies scholar Eve Tuck that “contaminants research” should go beyond documentation of damage to necessarily consider the addressing of its source with inclusion of the agency of those affected, communication of population results to individuals and their communities warrants greater attention.61
Beyond ethical considerations that guide much of the decisions on report-back, logistical issues can also be obstacles for researchers to communicate results. Some survey participants mentioned losing contact with subjects, pointing to the broader issue of lack of guidance on approaches that would allow for follow up with subjects. For example, this guidance could cover the ins and outs of establishing good working relationships with subjects that naturally open a clear channel for report-back.62 However, it can also be considered that lack of contact with subjects is symptomatic of scientists’ lack of motivation to engage with subjects in this way.
Our study suggests that researchers conduct report-back of biomonitoring results to a greater degree in practice than what is reflected in their publications. Decisions to conduct report-back and communication strategies are also informed by common debates and ethical considerations, indicating greater awareness on these issues among researchers than what can be gauged from their articles. In light of these findings, it is paramount that researchers are encouraged to share report-back approaches and experiences within their publications for the benefit of other researchers and IRBs. This begins with understanding whether researchers deem publications and journals in this field to be useful platforms for this type of discussion, a topic briefly explored through the survey component of this study. Furthermore, it is necessary to gain insight into potential constraints researchers face in publishing this type of information by investigating specifications and review processes of journals in environmental health and toxicology and attitudes of their editors on the appropriateness and value of publishing these topics within articles. Finally, we recommend the mainstreaming of guidance documents that compile evidence-based strategies on report-back. For example, the handbook produced by the Silent Spring Institute offers effective methods for reporting results, and crucially recommends inclusion of report-back evaluation that serves to improve knowledge on ethical practices in biomonitoring report-back and provide clarity on key ethical dilemmas.63
Conclusion
HBM has changed the way we look at human interactions with the environment and the ways in which chemical pollution affects our bodies. However, opportunities presented by this technology must be explored with caution. Research that utilizes HBM can sometimes inadvertently label populations as deprived, damaged, or legacies of historical and present abuses, even when intended to bring about positive change.64 This study indicates the need for readily available, evidence-based guidance on report-back of biomonitoring results to ensure that research in environmental health benefits affected populations through promoting greater awareness on pollutants and actions to mitigate exposure. Based on the findings from this study, our team will ensure our report-back approach is documented and reflected within future publications to contribute to the evidence base and share our experiences with the wider international community. In this regard, our broader research team will continue to work closely and directly with local communities to connect communication of breast milk biomonitoring results with consideration of alternative solutions, in an effort to pursue the communities’ right to a safe and healthy living environment while protecting and promoting the human right to breastfeeding.
Acknowledgments
We would like to thank our Ecuadorian researcher and community partners as we continue to determine and pursue best practices for ethical research, and our survey participants for sharing their perspectives on biomonitoring report-back.
Alyssa Mari Thurston is an MSc public health student at the London School of Hygiene and Tropical Medicine, London, UK.
Federico Andrade-Rivas, MPH, is a PhD student at the Global Health Research Program, School of Population and Public Health, University of British Columbia, Canada.
Jerry M. Spiegel, MA, MSc, PhD, is co-director of the Global Health Research Program at the School of Population and Public Health and Professor at the Liu Institute for Global Issues, University of British Columbia, Canada.
Please address correspondence to Jerry M. Spiegel. Email: jspiegel@mail.ubc.ca.
Competing interests: None declared.
Copyright © 2019 Thurston, Andrade-Rivas, and Spiegel. This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- D. Shelton, Human rights, health and environmental protection: Linkages in law and practice, A Background Paper for the WHO, Health and Human Rights Working Paper Series No. 1 (2002); P. J. Landrigan, R. Fuller, N. Acosta, et al., “The Lancet Commission on pollution and health,” The Lancet 391/10119 (2018), pp. 462–512; W. A. Suk, H. Ahanchian, K. Asante, et al., “Environmental pollution: An under-recognized threat to children’s health, especially in low- and middle-income countries,” Environmental Health Perspectives 124/3 (2016), pp. A41–A45.
- V. Yusa, M. Millet, C. Coscolla, et al., “Occurrence of biomarkers of pesticide exposure in non-invasive human specimens,” Chemosphere 139 (2015), pp. 91–108.
- World Health Organization, Human biomonitoring: facts and figures (Copenhagen: WHO Regional Office for Europe, 2015), pp. 1–88; A. Alves, A. Kucharska, C. Erratico, et al., “Human biomonitoring of emerging pollutants through non-invasive matrices: state of the art and future potential,” Analytical and Bioanalytical Chemistry 406 (2014), pp. 4063–4088; K. Sexton, L. L. Needham, and J. L. Pirkle, “Human biomonitoring of environmental chemicals: Measuring chemicals in human tissues is the “gold standard” for assessing people’s exposure to pollution,” American Scientist 92/1 (2004), pp. 38–45.
- R. Morello-Frosch, J. G. Brody, P. Brown, et al., “Toxic ignorance and right-to-know in biomonitoring results communication: a survey of scientists and study participants,” Environmental Health 8/6 (2009); J. W. Nelson, M. K. Scammell, R. G. Altman, et al., “A new spin on research translation: The Boston Consensus Conference on Human Biomonitoring,” Environmental Health Perspectives 117/4 (2009), pp. 495–499.
- J. M. Spiegel, J. Breilh, and A. Yassi, “Why language matters: Insights on the social determination of health through North-South collaborative research,” Globalization and Health 11/1 (2015), pp. 9–26; J. Breilh, “La determinación social de la salud como herramienta de transformación hacia una nueva salud pública (salud colectiva). [Social health determination as a tool of transformation towards a new public health (community health)],” Rev. Fac. Nac. Salud Pública 31/Suppl. 1 (2013), pp. S13-S27;.
- Canadian Institutes of Health Research (CIHR), Natural Sciences and Engineering Research Council of Canada (NSERC), Social Sciences and Humanities Research Council of Canada (SSHRC), 2014. Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. Ottawa, Canada: Secretariat on Responsible Conduct of Research; E. Morton Ninomiya and N. J. Pollock, “Reconciling community-based Indigenous research and academic practices: Knowing principles is not always enough,” Social Science & Medicine 172 (2017), pp. 28-36.
- D. Shelton (see note 1); B. M. Meier and M. Labbok, “From the bottle to the grave: Realizing a human right to breastfeeding through global health policy,” Case Western Reserve Law Review 60/4 (2010), pp. 1073–1142; C. J. Chantry, A. Eglash, and M. Labbok, “ABM position on breastfeeding–revised 2015,” Breastfeeding Medicine 10/9 (2015), pp. 407–411.
- A. Cordner and P. Brown, “Moments of uncertainty: Ethical considerations and emerging contaminants,” Sociological Forum 28/3 (2013), pp. 469–494; R. G. Stahl, T. S. Blingman, A. Guiseppi-Elie, et al., “What biomonitoring can and cannot tell us about causality in human health and ecological risk assessments,” Human and Ecological Risk Assessment 16 (2010), pp. 74–86; Morello-Frosch et al. (see note 4).
- Ibid.
- Morello-Frosch et al. (see note 4); J. G. Brody, R. Morello-Frosch, P. Brown, et al., ““Is it safe?” New ethics for reporting personal exposures to environmental chemicals,” American Journal of Public Health 97/9 (2007), pp. 1547–1554.
- J. L. Ohayon, E. Cousins, R. Morello-Frosch, et al., “Researcher and institutional review board perspectives on the benefits and challenges of reporting back biomonitoring and environmental exposure results,” Environmental Research 153 (2017), pp. 140–149.
- Ohayon et al. (see note 11); Morello-Frosch et al. (see note 4); M. Arendt, “Communicating human biomonitoring results to ensure policy coherence with public health recommendations: analysing breastmilk whilst protecting, promoting and supporting breastfeeding,” Environmental Health 7/Suppl. 1 (2008); J. S. LaKind, C. M. Berlin, J. L. Stokes, et al., “Lifestyle and polybrominated diphenyl ethers in human milk in the United States: A pilot study,” Toxicological and Environmental Chemistry 90/6 (2008), pp. 1047–1054.
- Ohayon et al. (see note 11); R. Washburn, “Measuring personal chemical exposures through biomonitoring: The experiences of research participants,” Qualitative Health Research 24/3 (2014), pp. 329–344; J. G. Brody, S. C. Dunagan, R. Morello-Frosch, et al., “Reporting individual results for biomonitoring and environmental exposures: lessons learned from environmental communication case studies,” Environmental Health 13/40 (2014); C. Adams, P. Brown, R. Morello-Frosch, et al., “Disentangling the exposure experience: The roles of community context and report-back of environmental exposure data,” Journal of Health and Social Behavior 52/2 (2011), pp. 180–196; R. G. Altman, R. Morello-Frosch, J. G. Brody, et al., “Pollution comes home and gets personal: Women’s experience of household chemical exposure,” Journal of Health and Social Behavior 49/4 (2008), pp. 417–435.
- S. R. Geraghty, J. C. Khoury, A. L. Morrow, et al., “Reporting individual test results of environmental chemicals in breastmilk: Potential for premature weaning,” Breastfeeding Medicine 3/4 (2008), pp. 207–213; N. Wu, M. D. McClean, P. Brown, et al., “Participant experiences in a breastmilk biomonitoring study: A qualitative assessment,” Environmental Health 8/4 (2009).
- UNEP, Global Chemicals Outlook–Towards Sound Management of Chemicals (UNEP, 2013); Landrigan et al. (see note 1); S. Naidoo, L. London, H-A. Rother, et al., “Pesticide safety training and practices in women working in small-scale agriculture in South Africa,” Occupational and Environmental Medicine 67/12 (2010), pp. 823–828.
- Morello-Frosch et al. (see note 4); R. Bhatia, B. Brenner, B. Salgado, et al., “Biomonitoring: what communities must know,” Race, Poverty and Environment 11/2 (2004), p. 56.
- Ohayon et al. (see note 11).
- Adams et al. (see note 13).
- E. A. Emmett and C. Desai, “Community First Communication: Reversing information
disparities to achieve environmental justice,” Environmental Justice 3/3 (2010), pp. 79–84; Morello-Frosch et al. (see note 4); D. Quigley, “Applying bioethical principles to place-based communities and cultural group protections: The case of biomonitoring results communication,” Journal of Law, Medicine and Ethics 40/2 (2012), pp. 348–358; Adams et al. (see note 13). - D. I. Saxton, I. Brown, S. Seguinot-Medina, et al., “Environmental health and justice and the right to research: institutional review board denials of community-based chemical biomonitoring of breast milk,” Environmental Health 14/90 (2015); R. Washburn, “The Social Significance of Human Biomonitoring,” Sociology Compass 7/2 (2013), pp. 162–179; P. Brown, J. G. Brody, R. Morello-Frosch, et al., “Measuring the success of community science: The Northern California Household Exposure Study,” Environmental Health Perspectives 120/3 (2012), pp. 326–331; Altman et al. (see note 13).
- Ohayon et al. (see note 11); Saxton et al. (see note 20); Cordner and Brown (see note
8); P. Brown, R. Morello-Frosch, J. G. Brody, et al., “Institutional review board challenges related to community-based participatory research on human exposure to environmental toxins: A case study,” Environmental Health 9/39 (2010), pp. 1–12; Morello-Frosch et al. (see note 4). - D. I. Shalowitz and F. G. Miller, “Communicating the results of clinical research to participants: Attitudes, practices, and future directions,” PLoS Medicine 5/5 (2008), pp. 714–720; Cordner and Brown (see note 8).
- Ohayon et al. (see note 11)
- Washburn (see note 20); S. A. Quandt, A. M. Doran, P. Rao, et al., “Reporting pesticide assessment results to farmworker families: Development, implementation, and evaluation of a risk communication strategy,” Environmental Health Perspectives 112/5 (2004), pp. 636–642; C. V. Fernandez, E. Kodish, and C. Weijer, “Informing study participants of research results: An ethical imperative,” IRB: Ethics and Human Research 25/3 (2003), pp. 12–19; M. D. Ramirez-Andreotta, J. G. Brody, N. Lothrop, et al., “Reporting back environmental exposure data and free choice learning,” Environmental Health 15/2 (2017); Brown et al. (ee note 21); Adams et al. (see note 13); Morello-Frosch et al. (see note 4); Wu et al. (see note 14); Shalowitz and Miller (see note 22); Brody et al. (see note 10).
- Ohayon et al. (see note 11); Ramirez-Andreotta et al. (see note 24); Brody et al. (see note 13); Brown et al. (see note 20); Adams et al. (see note 13).
- Quigley (see note 19).
- Ohayon et al. (see note 11); Brody et al. (see note 13); Brown et al. (see note 20); Wu et al. (see note 14); Altman et al. (see note 13); Geraghty et al. (see note 14); Shalowitz and Miller (see note 22).
- Ohayon et al. (see note 11); Cordner and Brown (see note 8); Morello-Frosch et al. (see note 4).
- Washburn (see note 20); A. D. Hernick, M. K. Brown, S. M. Pinney, et al., “Sharing unexpected biomarker results with study participants,” Environmental Health Perspectives 119/1 (2011); Brody et al. (see note 10); Quandt et al. (see note 24); Fernandez et al. (see note 24).
- Ohayon et al. (see note 11); Emmett and Desai (see note 19).
- Arendt (see note 12).
- Hernick et al. (see note 29); Morello-Frosch et al. (see note 4); Shalowitz and Miller (see note 22).
- Morello-Frosch et al. (see note 4).
- Brody et al. (see note 13).
- Brody et al. (see note 13); Hernick et al. (see note 29); Altman et al. (see note 13).
- L. Claudio, J. Gilmore, M. Roy, et al., “Communicating environmental exposure results and health information in a community-based participatory research study,” BMC Public Health 18/784 (2018).
- Fernandez et al. (see note 24).
- Ohayon et al. (see note 11); T. J. Downs, L. Ross, D. Mucciarone, et al., “Participatory testing and reporting in an environmental-justice community of Worcester, Massachusetts: a pilot project,” Environmental Health 9/34 (2010); R. L. Dunn and G. B. Carey, “Developing a biomonitoring educational pamphlet for potential participants in a breast milk biomonitoring study,” Journal of Human Lactation 26/2 (2010), pp. 183–186; Brody et al. (see note 10); Fernandez et al. (see note 24).
- Adams et al. (see note 13); Hernick et al. (see note 29); Emmett and Desai (see note 19); LaKind et al. (see note 12); Wu et al. (see note 14); WHO, Fourth WHO-Coordinated Survey on Human Milk for Persistent Organic Pollutants in Cooperation with UNEP–Guidelines for Developing a National Protocol, (Geneva: WHO, 2007).
- Brody et al. (see note 13); Downs et al. (see note 38); Dunn et al. (see note 38).
- Hernick et al. (see note 29); Wu et al. (see note 14); Shalowitz and Miller (see note 22).
- Cordner and Brown (see note 8); Morello-Frosch et al. (see note 4).
- H. Arksey and L. O’Malley, “Scoping studies: Towards a methodological framework,” International Journal of Social Research Methodology 8/1 (2005), pp. 19–32; D. Levac, H. Colquhoun, and K. K. O’Brien, “Scoping studies: advancing the methodology,” Implementation Science 5/69 (2010).
- Moher, A. Liberati, J. Tetzlaff, et al., “Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement,” Annals of Internal Medicine 151/4 (2009), pp. 264–269.
- Ohayon et al. (see note 11).
- X. Rojas-Squella, L. Santos, W. Baumann, et al., “Presence of organochlorine pesticides in breast milk samples from Colombian women,” Chemosphere 91 (2013), pp. 733–739; J. Wasser, T. Berman, L. Lerner-Geva, et al., “Biological monitoring of Persistent Organic Pollutants in human milk in Israel,” Chemosphere 137 (2015), pp. 185–191.
- Rojas-Squella et al. (see note 46).
- Ibid.
- Rojas-Squella et al. (see note 46); Wasser et al. (see note 46); C. Guerranti, G. Perra, S. Corsolini, et al., “Pilot study on levels of perflurooctane sulfonic acid (PFOS) and perflurooctanoic acid (PFOA) in selected foodstuffs and human milk from Italy,” Food Chemistry 140 (2013), pp. 197–203; P. O. Darnerud, M. Aune, L. Larsson, et al., “Levels of brominated flame retardants and other persistent organic pollutants in breast milk samples from Limpopo province, South Africa,” Science of Total Environment 409 (2011), pp. 4048–4053; R. D. Behrooz, A. Esmaili-Sari, F. E. Peer, et al., “Mercury concentration in the breast milk of Iranian women,” Biological Trace Element Research 147 (2012), pp. 36–43; S. Gebremichael, T. Birhanu, and D. A. Tessema. “Analysis of organochlorine pesticide residues in human and cow’s milk in the towns of Asendabo, Serbo and Jimma in South-Western Ethiopia,” Chemosphere 90 (2013), pp. 1652–1657.
- Rojas-Squella et al. (see note 46).
- Guerranti et al. (see note 49); Darnerud et al. (see note 49); Behrooz et al. (see note 49); Gebremichael et al. (see note 49).
- Ohayon et al. (see note 11); Cordner and Brown (see note 8).
- LaKind et al. (see note 12).
- Ohayon et al. (see note 11); Cordner and Brown (see note 8); Morello-Frosch et al. (see note 4); Brody et al. (see note 10); Stahl et al. (see note 8).
- Brody et al. (see note 13).
- Dunn et al. (see note 38); Ohayon et al. (see note 11); Arendt (see note 12) LaKind et al. (see note 12).
- Ohayon et al. (see note 11).
- Adams et al. (see note 13); Quandt et al. (see note 24).
- A. Yassi, J. Breilh, S. Dharamsi, et al., “The Ethics of Ethics Reviews in Global Health Research: Case Studies Applying a New Paradigm,” Journal of Academic Ethics 11/2 (2013), pp. 83–101; A. Costello and A. Zumla, “Moving to research partnerships in developing countries,” BMJ 321 (2000), pp. 827–829; J. M. Spiegel, J. Breilh, and A. Yassi (see note 5).
- A. R. Coates, S. del Pino Marchito, and B. Vitoy, “Indigenous child health in Brazil,” Health and Human Rights Journal 18/1 (2016), pp. 221–234.
- E. Tuck, “Suspending damage: A letter to communities,” Harvard Educational Review 79/3 (2009), pp. 409–427.
- Downs et al. (see note 38).
- S. C. Dunagan, J. G. Brody, R. Morello-Frosch, et al., When pollution is personal: Handbook for reporting results to participants in biomonitoring and personal exposure studies (Newton, MA: Silent Spring Institute, 2013).
- Tuck (see note 61).
Appendix
Survey questions
- Did your team directly communicate results of contamination levels to research subjects?
- Yes
- No
- If results were communicated, how (e.g., report, brochure, workshop) and to whom (e.g., mothers of community involved) was this conducted?
- What type of results were reported to participants?
- Individual-level results (samples analyzed and results reported individually)
- Aggregate-level results (samples analyzed individually and group results reported)
- Pooled results (samples mixed for analysis and single result reported)
- Other (please specify)
- Why did your team choose this method of communication?
- In retrospect, would you have followed any other method? Why?
- Was your experience in communicating results to research subjects reported anywhere?
- What were the considerations behind communicating or not communicating results to research subjects?
- Academic journals in environmental health-related fields (e.g. toxicology and biomonitoring) are a useful platform to discuss ethical concerns regarding HOW contamination levels are communicated to research subjects.
- Strongly agree
- Agree
- Neutral
- Disagree
- Strongly disagree
- In contamination studies, whom do you feel is best positioned to communicate research findings to research subjects?
- Research team
- Municipal/community worker
- Health care worker
- Other (please specify)
- In your experience and within your field, what are the challenges to results communication and best practices for disclosure?
