Matthew M. Kavanagh, Jennifer Cohn, Lynette Mabote, Benjamin Mason Meier, Brian Williams, Asia Russell, Kenly Sikwese, Brook K. Baker
Health and Human Rights 17/1
Published June 4, 2015
Abstract
Recent years have seen significant advances in the science of using antiretroviral medicines (ARVs) to fight HIV. Where not long ago ARVs were used late in disease to prevent sick people from dying, today people living with HIV can use ARVs to achieve viral suppression early in the course of disease. This article reviews the mounting new scientific evidence of major clinical and prevention ARV benefits. This has changed the logic of the AIDS response, eliminating competition between “treatment” and “prevention” and encouraging early initiation of treatment for individual and public health benefit. These breakthroughs have implications for the health-related human rights duties of States. With medical advance, the “highest attainable standard” of health has taken a leap, and with it the rights obligations of States. We argue that access to early treatment for all is now a core State obligation and restricting access to, or failing to provide accurate information about, it violates both individual and collective rights. In a context of real political and technical challenges, however, in this article we review the policy implications of evolving human rights obligations given the new science. National and international legal standards require action on budget, health and intellectual property policy, which we outline.
Introduction
Recent years have seen significant advances in the science of using antiretroviral medicines (ARVs) to fight HIV. Where not long ago ARVs were used late in disease progression to prevent sick people from dying, today people living with HIV can effectively achieve viral suppression early in the course of disease through initiation of antiretroviral therapy (ART). Mounting evidence shows that early suppression provides major clinical benefits and prevents HIV transmission. Newer medicines have better enabled large-scale rollout of early ART in resource-constrained environments. This has changed the logic of the AIDS response, eliminating competition between “treatment” and “prevention” programs and making models of epidemiologic control in the near term increasingly credible.
These breakthroughs have implications for the health-related human rights duties of states and international bodies. Waves of HIV-related human rights challenges at the national and international level have helped establish strong (but imperfect) norms against discrimination and coercion and compelled States to prevent imminent death of citizens. Compliance with these basic expectations, however, is no longer sufficient—with medical advances, the “highest attainable standard” of health has taken a qualitative leap, and with it, rights-based obligations of States to provide early access to ART.
ARVs have long been considered an essential medicine, and thus part of the State’s “core minimum” obligation under the right to health.1 The last decade has brought vast improvements in the basic availability of ART. While many argue that the definition of “essential medicine” is insufficiently developed, WHO lists ARVs on its essential medicines list as among the “most efficacious, safe and cost-effective medicines for priority conditions. Priority conditions are selected on the basis of current and estimated future public health relevance, and potential for safe and cost-effective treatment.”2 ARVs are widely available, with clinical programs reaching many—though clearly not all—through a combination of domestic and international health programming. We argue that while this basic availability and non-discriminatory access were the core questions earlier in the epidemic, human rights norms now can and must speak to evolving science that begs the question of how, when, and for what purpose people should be guaranteed access. Highlighting the evolution of the right to health to reflect scientific changes in the “highest attainable standard of health,” access to early ART—the option to start ART immediately upon diagnosis and after an informed voluntary decision—is now part of a core human rights obligation. While States may differ on exactly how to implement their right to health obligations, the new evidence on ARVs shows that denying access to early ART violates the rights of people living with HIV and AIDS [PLWHAs], as does telling PLWHAs that there is a CD4-test-based “right” time to start ARVs, which has been recent practice. Delaying individual access to early treatment in turn also violates the collective right of disease prevention, with early ART shown to prevent the onward transmission of HIV.
As WHO and dozens of countries review their HIV treatment guidelines and practices, there is a particular gap between countries in the North (which increasingly provide early ART) and the South (which largely do not). We argue below that rationing early access to ART now cannot be justified by medical evidence, and that greater attention to the human rights implications of this new evidence is needed.
The political and technical challenges to implementing early ART for all in resource-poor settings are real—communities are already experiencing them—and a public health approach remains important. We argue that common health system weakness and resources-based justifications for rationing ART, however, are insufficient responses to rights-based claims for early ART. Increasingly, failure to fulfill core duties related to the right to health opens States to challenge in national courts—as seen recently in South Africa, Kenya, Uganda, Brazil, India, and many other countries—as well as in international human rights bodies.3 In this context, the question of what constitutes fulfillment has immediate significance.
New science of antiretroviral medicine
Just a few years ago, delivery of ART in resource-constrained environments centered on treating significantly immunocompromised people in order to prolong their lives. With this focused goal, programs were oriented toward late-stage initiation of therapy due to both delayed diagnosis and significant ARV toxicities such as lactic acidosis, peripheral neuropathy, and lipodystrophy. Concerns over treatment failure, resistance, and limited capacity of systems also oriented providers toward late ART initiation. Asymptomatic patients were at best monitored for deterioration of immune status or at worst sent home to return when sicker. New data and technologies, however, have changed the value proposition of ART.
Studies have long provided strong evidence that ART initiation below CD4 count of 350cells/mm3 holds significant benefits. New evidence since 2010 supports earlier/immediate initiation leading to both population- and individual-level benefits. In May, 2015 the START study, a randomized clinical trial of over 4,000 participants in 35 countries, was stopped more than a year early when evidence showed that immediate ART was associated with a 53% reduction in serious illness or death compared to waiting until 350 CD4.4 High income-country cohorts have shown those with a CD4>750/μL have a lower rate of AIDS-defining illness than individuals in the 500/μL-749/μL range, including in those virologically suppressed on ART; and demonstrate longer AIDS-free survival when ART is initiated at CD4 500/μL as compared to CD4 350/μL.5 Lower rates of drug resistance mutations have been shown for those failing on ART who were initiated at CD4>350/μL.6 Increasing evidence also suggests that initiation of ART as soon as possible can prevent cardiovascular events, neurological damage, and other non-AIDS co-morbidities.7 There is also evidence that initiating treatment early significantly enhances recovery of immune system to high CD4 levels and reduces HIV reservoirs, which may be important for curative therapy in the future.8 In low- and middle-income countries (LMICs) the HPTN-052 randomized controlled trial has shown that initiating ART at CD4>350/μL leads to significant reductions in clinical events, including AIDS-defining illnesses and TB, and a systematic review reveals a decrease of more than 50% in TB for patients initiated on ART with CD4>350/μL.9 Finally, preliminary results from the TEMPRANO study, a randomized controlled trial (RCT) of 2,056 patients in Cote d’Ivoire, show that immediate ART initiation (compared to ART initiation in accordance with WHO guidelines) reduces severe HIV morbidity or all-cause mortality, including in patients with a baseline CD4>500/μL.10 Treatment does not fully restore health in all individuals, and patients might choose to delay for a variety of individual reasons.11 However, taken together, these findings support important benefits for the individual in initiating ART early in the course of disease. Studies now provide no evidence of a CD4 count level above which it is “safe” to delay ART initiation.12
Meanwhile, there is clear evidence of population-level prevention benefits of early ART. Prospective cohorts in sub-Saharan Africa and a large randomized clinical trial have shown the benefits of ART for prevention of HIV transmission in serodiscordant couples.13 The RCT demonstrated a 96% reduction in transmission, and a prospective cohort in Uganda showed no HIV transmissions among couples where the HIV-positive partner was virally suppressed.14 In high-burden settings, one study showed a 1.4% decline in risk of acquiring HIV for every 1% increase in ART coverage.15 Population-level transmission of HIV-related TB is also decreased, with sub-Saharan African modeling showing a 48% reduction with immediate ART.16
Cost and impact studies also support early initiation. Already, ART has resulted in life expectancy gains of over 11 years in hyper-endemic settings.17 Earlier treatment has been projected to be cost saving in South Africa, as ART costs are outweighed by health benefits and averted infections, and are cost-effective in Uganda, India, and other countries.18 Economists have shown far-reaching economic gains to patients and their households, employers, and societies attributable to ART.19
Today’s drug toxicity profile has also improved, which makes early treatment far more acceptable as health trade-offs diminish.20 Improved medicines and diagnostics could make ART even more tolerable, durable, effective, and perhaps even cheaper. New medicines in the pipeline making use of long-lasting formulations, nanotechnology, and other advances promise fewer side effects, increased efficacy, eased dosing schedules, and reduced infrastructure needs.21 Meanwhile, shifting models of care (including task shifting to less specialized health worker cadres) have been shown to result in equivalent or even improved care outcomes while decreasing costs.22
In sum, the science of ART impact and delivery is bringing a new paradigm in the HIV response, and with it important human rights implications.
Updating human rights conceptions
Important work on human rights in the context of HIV treatment has established a strong, though not universal, normative framework for health policy. Early work tackled the dilemmas of governmental and societal discrimination and exclusion on the one hand, and coercive control of public health threats on the other.23 The basic lack of an AIDS treatment response, first in the North and later in the South, was framed as a violation of core human right to life. Even as treatment and testing began to roll out, human rights also formed the basis of a challenge to coercive public health measures. Forced testing, quarantines, coercive treatment efforts, criminalization, and compelled sterilization featured in the early response of many countries—and all have come to be seen as illegitimate under a broad, albeit incomplete, agreement that liberty and choice for people living with HIV is fundamental to human rights and public health. Human rights concerns were also mobilized to demand essential “due process”-type rights of people living with HIV to participate in decision making and of disfavored and neglected populations not to be excluded from services. These liberty interests seem increasingly rooted in the HIV response, with the understanding that human rights promotion is “inextricably linked” to public health advancement.24
Human rights discourse, however, has too often remained normatively limited to individual freedoms, which are insufficient to deal with the new complexities of scientific evidence. There has been too little discussion of the rights of HIV-positive people as a whole or the rights of HIV-negative people who share their communities. In this context, the human right to have an infectious disease treated was pitted against the human right to remain disease-free, which legitimized a discussion over whether to shift global priorities from treatment towards prevention.25 Today, however, the new science of ART makes this individualism untenable: group access to treatment is group prevention.
In this context, collective rights to health and individual freedoms need not stand in opposition. A collective enjoyment of public health should be understood today as inextricably linked to the individual right to health, with public health addressing collective determinants of health outside the control of the individual. Facilitating both prevention and treatment, recent scientific advances alleviate the tension between the collective goal of preventing the spread of disease and the individual goal of treating the dying individual.26 Today’s human rights subject must be understood as a more realistic rights-bearing person who experiences both reciprocal entitlements and responsibilities for themselves, their partners, and their community. Only in this context can we begin to answer the key contemporary question: what must States do to fulfill their human rights obligations on HIV treatment and prevention today? The simple existence of a national HIV treatment program, refraining from coercion, involving communities, and putting in place ethical protocols are all necessary. But we argue that this is not sufficient given new medical evidence. Now that it is clear that early viral suppression positively impacts the health of entire communities, how must States respond?
The continued coercive potential of public health cannot be ignored—new evidence of the prevention benefits of ART could increase threats, especially for key populations.27 The opportunity should not deter early access but instead intensify the need for solutions, and indeed, experts have shown that putting structures in place to guard against such rights violations, while universalizing access, costs an average of just 1.5% of HIV budgets.28
Elements of a human-rights based response to new ARV science
The right to health has a strong and growing basis in international law and domestic constitutions. Today, every country in the world is party to at least one treaty that enshrines the right to health.29 The seminal provision, Article 12 of the ICESCR, provides for the “right of everyone to the enjoyment of the highest attainable standard of physical and mental health.” This highest attainable standard is to be “progressively realized” over time and within a State’s available resources, but there remains a set of “core minimum” obligations that takes immediate effect, including a right of access to essential medicines.30 A majority of national constitutions now also guarantee the right to health.31 Early examples in South Africa and Brazil saw the right to health mobilized to open the floodgates to ARVs—and to a burgeoning enforcement movement, led by activists and engaging national courts, to present claims for a right of access to HIV treatment. From Colombia to Kenya to India, courts in just the last few years have ordered policy overhauls to ensure access to medications; in countries like Brazil and Costa Rica, tens of thousands of individual suits are filed each year for access.32
Access to essential medicines is now firmly entrenched as part of the core minimum obligations both internationally and in many domestic jurisprudences, but little has been concluded on the question. What are the implications now that ART is not only life-saving for the severely ill but beneficial for the public’s health?
The concept of a minimum core is not universally embraced.33 Nonetheless, it remains influential in shaping how national governments and international organizations understand their obligations on issues ranging from medicines to the structuring of health systems and “can assist as an object of interpretive agreement—or disagreement—around claims for socioeconomic protection.”34 The minimum core is not a decontextualized claim to all things for all people. Instead, it is a lens through which to understand and consider what must be provided immediately toward the full realization of the right to health. New evidence on ARVs unsettles this understanding. WHO 2013 ART guidelines, for example, argue that human rights “should guide the revision of national treatment policies.”35 Yet the guidelines limit this inquiry exclusively to a much narrower conceptualization of human rights than we believe is appropriate. Failing to bring in the duty of States to “fulfill” a core minimum and to interrogate what that means in the context of new scientific evidence suggests that human rights norms would be satisfied by simple provision of ART to some people, sometimes, on a non-discriminatory basis, even if it is based on outmoded medical evidence. We argue it does not and believe the framework articulated by the CESCR provides a strong basis to understand why denying access to early ART now violates human rights norms.
The UN Committee on Economic, Social and Cultural Rights’ General Comment 14 clarifies that States are obligated to use all tools at their disposal to progressively realize the right to health—budget, policy-making, and planning.36 The AAAQ matrix—availability, accessibility, acceptability, and quality—provides a framework with which to analyze these State obligations. Our effort here aims to articulate how the minimum core access to medicines obligation has changed. Bringing together collective and individual conceptualizations of human rights helps us understand that the “highest attainable standard” for PLWHAs now includes early viral suppression through ART. Human rights claims can be made, both on behalf of individuals and communities, for access to the benefits of early ART.
Table 1 details our analysis of specific core State duties that emerge from this framework.
| Table 1: Minimum core obligations of states for early ART | ||
| Core State duties | Implications of new science | |
| Availability | National guidelines provide scientifically appropriate treatment guidelines for essential medicine | · Adaptation of national guidelines to at least reflect WHO 2013 as a minimum.· Obligation to consider universal, voluntary early ART for all as strategy to reach viral suppression, the new highest attainable standard. |
| Donors and implementing states share duty to ensure adequate and sustained funding needed to purchase medicines and provide the health services to reach all PLWHAs |
|
|
| National plan to make early ART possible |
|
|
| Sufficient health workforce |
|
|
| Accessibility | ||
| Information accessibility | All PLWHAs are fully informed in their medical decision-making. |
|
| Physical accessibility | ARVs are available to people regardless of geographic location. |
|
| Non-discrimination | Health services are equally accessible to all. |
|
| Affordability | Antiretroviral and opportunistic-infection drugs are affordable for the lowest-income individuals. |
|
| Acceptability(meet ethics and custom) | Treatment and prevention programs meet medical ethics and are culturally appropriate. |
|
| Quality | Medicines meet contemporary standards of care. |
|
| Quality of health services sufficient for real opportunity to be healthy. |
|
|
Health policy and human rights
National guidelines, planning, and policy-making
Setting guidelines for ART remains a core State function, even as WHO provides recommendations. Today, an HIV-positive person walking into a clinic in Los Angeles or Paris would be advised under national guidelines to start ART immediately, regardless of their CD4 count. The same person in Jakarta or Kampala might be considered “ineligible” to start ART if their CD4 count is above a set threshold (350 and 500 respectively).37 This contradiction is problematic and the availability standard requires that countries re-examine their guidelines in the light of new science.
During the writing of new recommendations in 2013, WHO found evidence of both individual benefit and collective prevention while finding no studies showing that earlier ART caused individual harm.38 It suggested new standards supporting expansion of ART to all people with CD4 counts below 500 cells/μL, as well as immediate ART for pregnant women, people in serodiscordant couples, those with active TB or hepatitis B, and children younger than five. Out of an estimated 35.3 million people living with HIV, 28.3 million would be eligible for treatment under this standard.39 The population-level prevention benefit is also dramatic: WHO estimates that early ART can reduce AIDS deaths and new HIV infections by 36-39% over the next 12 years.40
WHO guidelines, however, are under review at the time of this publication, and the 2013 guidance should provide a floor for the realization of a core minimum obligation. Insofar as having a coherent plan is part of the basic core minimum obligations of a right to health, a State’s obligation with respect to its people is to evaluate the evidence and national epidemiology. States must plan to help as many people as possible fully realize their right to the highest attainable standard of health.41 Many countries have decided that all PLWHAs should have the option to initiate ART immediately—including the US, France, Spain, Brazil, and Korea, among others. For those who have not, a human rights-based review should call into question the decision to maintain a particular CD4 count as a barrier to accessing ART.
Duty-bearers might look at the evidence and decide not to implement immediate ART, but they have an obligation pursuant to the right to health to consider this in national guidelines. In the past, the default setting has been to maintain or slightly raise the CD4 count at which ART initiation is allowed without actually assessing it as a barrier to access with human rights implications. The challenges of implementing immediate ART are significant, including how to effectively support adherence in large numbers of asymptomatic people. Through the lens of the collective and individual rights discussed above, however, it is increasingly clear that sending HIV+ people home without ART (only to return when they are sicker) is a human rights violation. This fact is underscored by research showing that the staging process for ART in lower-income countries loses far more patients than it reaches—only between 17% and 25% of those diagnosed with HIV in Sub-Saharan Africa ever initiate ART.42 Many of those sent home because their CD4 count was too high will die before re-engaging with health services to access ART.
Meanwhile, implementing existing policy has the potential to create a convergence of liberty and entitlement challenges. Restricting immediate ART to serodiscordant couples, for example, could result in discrimination if health care service providers become gatekeepers. Guidelines restricting ART prescribing powers to only the highest cadre of health workers, especially where there are workforce shortages, can be understood not only as disproven policy but as a significant restriction on the availability and accessibility of ART.43
Duty bearers are further under a human rights obligation to consider the social context within which policy operates. Given the collective prevention benefits of ART, denying people living with HIV the option to start ART has clear implications for their partners and communities. Despite decades of behavior change efforts, rates of condom use are still relatively low in many communities, and coercive sex (including within marriage) is still all too common. The risk of HIV for key populations, including men who have sex with men, sex workers, and drugs users is driven by structural factors of social marginalization, economic inequities, and stigma. Significant population health capabilities, especially for the most marginalized, depend on the ability of others to access ART. The “community viral load” structures the relative risk experienced so differently by members of the same community.44
Information and rights protections for PLWHA and communities
In addition to health policy on access, States have a human rights obligation to ensure populations are educated about essential health issues and must respect the right to correct and impartial health information.
The clearest information implication of the new science is on HIV transmission: the HPTN052 study made clear the prevention benefits of ART. Understandings of collective rights help us see that States have a clear obligation, especially in high-burden populations, to make both PLWHAs and communities aware that an undetectable viral load is a powerful prevention tool. Nonetheless, rights to privacy and bodily integrity still require that PLWHAs control their own medical decisions—partners have no right to see medical records, and communities have no right to coerce PLWHAs into treatment.
Providing information on the clinical benefits of ART is less clear, however, given remaining uncertainty over the exact cost-benefit calculation. What is clear is that telling people living with HIV that there is a “right” CD4 count at which to start ART is no longer tenable. Since this has been the prevailing public health practice for decades, States are under an obligation to disseminate new information. Instead, PLWHAs must be given the opportunity to consider the risks and benefits and make decisions in light of individual and collective realities after understanding (a) the considerable evidence that early ART has shown significant health benefits and clear prevention benefits; and (b) the limited side effects and medical uncertainties of ART. They must also be given the opportunity to decline to start ARVs, and some informed people are likely to do so for reasons ranging from readiness to adhere to marginalization and stigma. Helping address those issues is core to the work of clinical practice of AIDS treatment, but it is the duty of States to ensure that this choice is meaningful and informed. Human rights hotlines, information campaigns, and other outreach techniques can help States ensure this double task of informing and providing choice.
Given the new science, viral load is the most critical piece of information PLWHAs need to understand their health, treatment, and the best way to prevent transmission. Providing at least annual viral load tests is thus a high priority for rights-based practice and central to information accessibility.
Financing and human rights
Perhaps the most important implication of global recognition of ARVs as essential medicines is that, as a part of the core minimum, they are not subject to progressive realization in financing immediate access for all. Claiming insufficient resources is not a legitimate defense to rights-based claims for access.
At first blush, this invocation of a core obligation to realize essential medicines for all can make such human rights claims seem unrealistic. Supporting claims that could bankrupt the health system is hardly a good strategy for PLWHA. Nor are human rights claims that ignore budget trade-offs particularly helpful.45 Real experience in this case, however, shows that neither is of sufficient concern to justify ignoring the rights implications of medical advances and continued rationing of ART, especially where early ART has been shown to be cost-saving.
High-income countries are largely able to afford immediate ART for all PLWHA, even while making what we argue below are poor public health decisions related to intellectual property. Low- and middle-income countries struggle more. Figure 1 describes what universal ART would cost compared to GDP in a set of the highest-burden countries. While a significant expense for all, the cost runs a gamut: for quite a few the cost falls in the 0.1-1.0% of GDP range, which is not inherently prohibitive; for others, the costs run far higher, threatening to exceed total spending on health. For none of these countries, however, are the claims we make divorced from resource considerations. There are four clear paths to ensuring sufficient and sustainable financing: reasonable budgets, international cooperation, effective program allocations, and use of intellectual property flexibilities to reduce drugs costs. To our knowledge, no country has exhausted these options. In the absence of these policy levers, refusing to fulfill the right to immediate access to ART cannot be justified.
Figure 1: Cost of ART in high-burden countries
Cost of universal ART as a proportion of GDP (%)
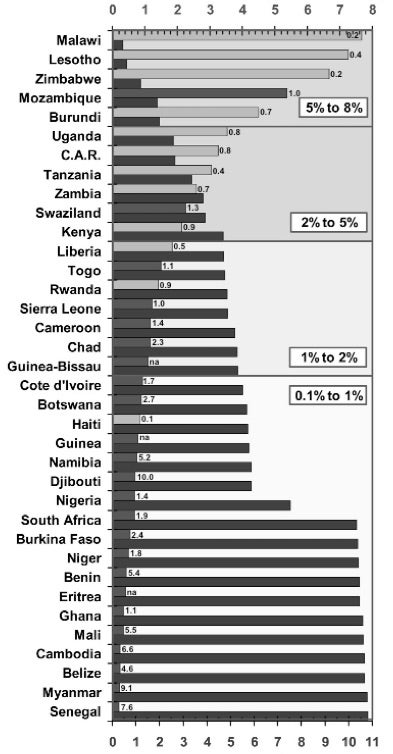 Cumulative cost (US$ Bn p.a.)
Cumulative cost (US$ Bn p.a.)
National and donor budgets
In recent years, many countries have substantially expanded their commitments such that domestic spending has now surpassed donor funding, though that includes “out of pocket” expenses and thus may overstate the degree to which States are meeting their obligation.46 Other countries have failed to do so, though insufficient funding of the health sector within an existing national budget is not a justification for failing to fulfill rights duties. This is not a call for unlimited funds for ART, or even for health, but instead a call for a case-by-case assessment of whether health budgets are reasonably allocated before deciding early ART is too costly. African countries have committed in the Abuja Declaration to allocate at least 15% of their national budgets to public health by 2015, though only 9 of 53 had achieved that goal in recent years.47 Activists and the legal complex have shown increasing willingness to challenge the failure to meet these standards, as seen recently when activists in Uganda challenged the maternal health budget in a much-watched case currently under appeal to the Supreme Court.48
Human rights instruments also make it clear that where States lack sufficient resources, other States have an obligation of international assistance and cooperation to ensure realization of core minimum obligations. Figure 1 shows that for some highly affected countries, the cost of universal ART would rise well above 2% of their total GDP per year, with countries like Malawi and Zimbabwe reaching above 5%. In this context, even strong budgetary allocations by national governments would still be insufficient. As Gostin et al. note, the human rights duties of wealthy states in such contexts is “one of the most inadequately understood obligations,” often confusing agreed-upon assistance obligations with charity.49 States have long highlighted HIV as a critical global priority and committed themselves to act, thus solidifying a binding obligation.50 Estimates of the gaps for high-burden countries have been well documented. However, for several years, donor funding for AIDS has been flat and has done little to close the funding gap, suggesting donor nations are not fulfilling their human rights obligations.51
Maximizing existing funding
An estimated US$19.1 billion was available for HIV from all sources in LMICs—which is clearly insufficient to meet all the health needs of PLWHAs and communities.52 There is, however, evidence that efforts can make HIV programs significantly more affordable in the long term and early treatment more affordable in the near term. The Clinton Foundation, for example, has shown that facility-level costs of treating a person living with HIV in Africa are as low as $200 per year, while Médecins Sans Frontières (MSF) implements large-scale ARV programs in Malawi for $237 per patient per year, with drugs forming two-thirds of the cost.53
These do not reflect the full costs to the health system, but they do suggest that significantly more people could access ART within existing funding through effective, lower-cost efforts (including community-based programs, as discussed below). In a few countries, it may even be possible to reach all PLWHAs with an offer of early ART within existing funding envelopes without undermining other priorities.
Continuing to support outmoded practices—including funding disproven interventions while claiming inability to afford proven ones—can also open States to challenge. This was the case in South Africa, where substantial funding was available for HIV but was not directed to ARVs, and in the US where activists brought legal challenge to state-financed disproven “abstinence-only” programs.54 Even where funding decisions are not as glaring as these, many States have not yet evaluated their public health decisions in light of new scientific evidence. While courts and rights bodies are generally loath to dictate budgets to national governments, they might be compelled, in cases where States are failing to make immediate ART available, to justify through rights claims—with evidence—budget decisions that do not leave sufficient funds for early ART.
Affordability and TRIPS flexibilities
Accessibility of medical care includes affordability. States have a duty to make use of all policy space at their disposal to ensure medicine costs are not a barrier to access. While ample evidence shows that maximalist intellectual property (IP) regimes drive up costs of essential medicines, there is little or no empirical evidence that they deliver the promised innovation, investment, or economic growth, especially in low- and middle-income countries.55 Instead, the countries that have been most effective at making medicines more affordable are those that have made use of flexibilities in global IP rules to enable generic production or importation.56
Since price is often the main barrier to ARV access, countries have both an opportunity and a core human rights obligation to enact, preserve, and make use of all available flexibilities in World Trade Organization agreements.57 These include using “compulsory licenses” to access generic versions of newer AIDS drugs, importing lower-priced drugs in “parallel” from nearby countries, and creating high standards of patent review that limit low-quality patents. Least-developed countries, which include many of the highest HIV burden countries, received an extended transition period and are not required to even issue patents on medicines for the foreseeable future. Unless all of these mechanisms have been used, high price of medicines will not be an adequate defense to human rights claims against States. Likewise, human rights obligations of high-income countries should prevent them from demanding that LMICs implement IP measures that negatively impact access to medicines, whether through free trade agreements or otherwise. The Global Commission on HIV and the Law has gone so far as to call not only for a cessation of such demands but a moratorium on the enforcement of medicines-related intellectual property rights (IPR).58
Human rights and the health system
Renewed conceptualization that can effectively take into account the evolution in medical evidence—and specifically new imperatives for ARV treatment to fulfill obligations for PLWHAs and their communities—have broader implications for the health system as a whole. Denying access to early ART can no longer be justified on medical grounds, which confers new obligations on states and international organizations, but such obligations for access to ART need not be disconnected from the broader health system. Health policy planners are increasingly recognizing that such “diagonal” approaches can avoid the pitfalls for purely vertical problems while ensuring the urgency and focus needed for pressing infectious disease response.59 PLWHA have needs that go well beyond ART and, in most settings, delivery of ART is dependent on many elements of the broader health system—from drug supply chains to frontline clinicians to HIV testing in antenatal care.
Ensuring individual access to ART as a human rights obligation, initiated regardless of CD4 count based on a patient’s informed decision, does not conflict with broader strategic public health goals. Challenges to ensuring effective programs are significant, and communities are already facing them. Indeed, realizing this right holds promise to improve the health system overall. Health workforce shortages, for example, must be overcome to allow early ART rebound, as the same doctors, nurses, and clinical officers providing general care also dispense and monitor ART.60 A human rights lens draws special attention to several specific areas in this context.
Equitable access to quality drugs regardless of geography
In addition to core health rights, there is a basic human right to benefit from the advancement of science, which is perhaps nowhere more important than early access to new medicines. Yet communities in the Global South, who need effective new treatments most, receive innovation last—often many years after the US and Europe. Important new HIV drugs like dolutegravir, which the US added to its guidelines in a rapid update, have potential for low-dose, long-acting formulations, and superior efficacy.61 Other drugs and diagnostics in the pipeline could make ART even more tolerable, durable, and effective with fewer side effects and reduced infrastructure needs.62 For asymptomatic patients, some of these newer drugs are likely to make the difference between whether early treatment is acceptable and accessible or not, with prevention implications for the public’s health. In the Global South, however, newer drugs are largely unavailable, not only because of cost but also because of government delays in guideline adoption and drug registration, and pharmaceutical manufacturer decisions to postpone or neglect registration in smaller and poorer countries. A human rights perspective draws attention to failures of States—and perhaps even more so, international institutions—in eliminating this delay as a human rights issue. There is no reason why drugs should be distributed last to those most in need, except failure of the market to incentivize drug registration in poor countries. This is a problem that can be addressed, and is exactly the type of collective action challenge that WHO can and should address. In the US, HIV became a transformative issue and rights claims drove the creation of new models of drug regulation that are now used to speed access to a wide variety of medicines.63 A similar sea change is needed globally to create new mechanisms to drive early access to essential medicines and realize the highest attainable standard of health. Doing so would cascade well beyond HIV. The WHO Prequalification Programme and Collaborative Registration Project are only partial solutions to these needs.
Building community-based models
Second, the “quality” element within the right to health framework also extends to the delivery of services. Without expecting that all health systems will achieve perfect outcomes, PLWHAs have a right to programs that are sufficiently staffed to support durable retention in quality care in order to achieve and maintain an undetectable viral load. It is increasingly clear that this requires innovative approaches that expand service delivery into the community where studies have shown non-inferior results—and significantly superior accessibility.64 Programs such as those piloted in southern Africa have empowered stable patients to join community-supported groups who collectively monitor wellbeing, distribute drug refills, and provide adherence support and education. Such models empower patients as rights-holders who can access not just the information but also the tools to support “health capability.”65 Governments and donors are often reluctant to support such efforts, considering them optional add-ons. The human rights conception described here, however, suggests differently. The obligation of States is not simply to make drugs available when people seek them out, but to give populations the capability to use these drugs to achieve individual health and collective prevention. As States weigh how to truly get all PLWHAs real access to ART before they become immunocompromised, rights-based claims will outweigh unfounded fears that drugs will end up on the black market or professional associations’ interest in monopolies over drug dispensing. Empowered patients and communities take care of each other and themselves; they are partners in the realization of the individual right to health and collective rights to public health.66 While this framework might be clearest with HIV treatment, a rights-based framework suggests similar imperatives for other infectious and non-communicable diseases wherever empowering patient groups and community-based care will conceivably expand access.
Conclusion: The future of enforcement
HIV has long been the locus of cutting-edge challenges to public health practices on human rights grounds. From Indiana to India, people living with HIV and their advocates have mobilized human rights norms to successfully challenge deadly denial of access to lifesaving drugs, as well as discrimination and coercive public health practices. ARVs are no longer just a tool to prevent death; viral suppression with newer medicine now enables a “normal” lifespan relatively free of crippling side effects and is one of the best prevention interventions available. We argue that, given the new science, States’ core minimum obligations now include access to early ART for both individual and collective benefit. Governments can now reasonably be expected to enable early viral suppression through human rights-based interventions, and this is information that must be shared with individuals. As the right to health is increasingly enforced, especially by PLWHAs, States should take careful notice. It is still up to States to do the difficult work of balancing budgets and health priorities, but with the qualitative leap in the science of ARVs, there is a new standard in the “highest attainable standard” of health against which these decisions will be judged.
Matthew M. Kavanagh is a fellow at the Leonard Davis Institute of Health Economics and Department of Political Science at the University of Pennsylvania, Philadelphia, PA, USA, and Senior Policy Analyst at Health Global Access Project, Philadelphia, PA, USA.
Jennifer Cohn is Medical Coordinator of the Médecins Sans Frontières Access Campaign, Geneva, Switzerland, and Assistant Professor of Infectious Disease at the Hospital of the University of Pennsylvania, Philadelphia, PA, USA.
Lynette Mabote is the Advocacy Team Leader at the AIDS and Rights Alliance of Southern Africa, Cape Town, South Africa.
Benjamin Mason Meier is Associate Professor of Global Health Policy at the University of North Carolina, Chapel Hill, NC, USA.
Brian Williams is Senior Research Fellow at the South African Centre for Epidemiological Modelling and Analysis, University of Stellenbosch, Stellenbosch, South Africa and the Wits HIV and Reproductive Health Institute, University of the Witwatersrand, Johannesburg, South Africa.
Asia Russell is the Executive Director of Health Global Access Project, Philadelphia PA, USA and Kampala, Uganda.
Kenly Sikwese is Coordinator of the African Community Advisory Board, Lusaka, Zambia.
Brook K. Baker is Professor of Law at Northeastern University and Senior Policy Analyst at Health Global Access Project, Boston, MA, USA.
Competing interests: None declared.
Copyright © 2015 Kavanagh et al. This is an open access article distributed under the terms of Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licences/by-nc/3.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original authors and source are credited.
References
- H. Hogerzeil et al., “Is access to essential medicines as part of the fulfilment of the right to health enforceable through the courts?,” Lancet 368, (2006): pp.305–11; R. Laing et al., “25 years of the WHO essential medicines lists,” Lancet 361/9370 (2003): pp.1723–29.
- WHO, The selection and use of essential medicines (Geneva: WHO, 2003).
- A. Yamin, “Promoting equity in health: What role for courts?” Health and Human Rights Journal 16/2 (2014); E. Friedman and L. O. Gostin, “Pillars for progress on the right to health,” Health and Human Rights Journal 14/1 (2012), pp.1–16.
- National Institutes of Health, “Antiretroviral treatment early improves outcomes for HIV-infected individuals,” (2015), Available at http://www.nih.gov/news/health/may2015/niaid-27.htm.
- A. Mocroft et al., “The incidence of AIDS-defining illnesses at a current CD4 count≥ 200 cells/µL in the post–combination antiretroviral therapy era,” Clinical Infectious Diseases 57/7 (2013), pp.1038–47; CAUSAL et al., “When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries,” Annals of Internal Medicine 154/8 (2011), p.509.
- J. Uy et al., “Initiation of HAART at higher CD4 cell counts Is associated with a lower frequency of antiretroviral drug resistance mutations at virologic failure,” JAIDS 51/4 (2009), pp.450–53.
- B. Marin et al., “Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy,” AIDS 23/13 (2009): pp.1743; J. A. Womack et al., “HIV infection and cardiovascular disease in women,” Journal of the American Heart Association 3/5 (2014); S. Spudich, “Neurologic complications of HIV infection,” Topics Antiviral Med 22 (2014), pp. 594–601.
- T. Le et al., “Enhanced CD4+ T-Cell recovery with earlier HIV-1 antiretroviral therapy,” New England Journal of Medicine 368/3 (2013): pp.218–30; L. Hocqueloux et al., “Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts,” Journal of Antimicrobial Chemotherapy 68/5 (2013): pp/1169–78.
- B. Grinsztejn et al., “Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection,” Lancet ID 14/4 (2014): pp.281–90; A. B. Suthar et al., “Antiretroviral therapy for prevention of tuberculosis in adults with HIV,” PLoS Medicine 9/7 (2012).
- C. Daniel et al., “Early ART and IPT (Temprano Trial),” (conference on retroviruses & opportunistic infections, Seattle, WA: IAS-USA, 2015).
- S. Deeks, S. Lewin, and D. Havlir, “The end of AIDS,” Lancet 382 (2013): pp.1525–33.
- Study Group on Death Rates, “Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells,” Lancet 376/9738 (2010): pp.340–45; A. N. Phillips et al., “Rate of AIDS diseases or death in HIV-infected antiretroviral therapy-naive individuals with high CD4 cell count,” AIDS 21/13 (2007): pp.1717–21; S. Grabar et al., “Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French hospital database on HIV,” AIDS 23/9 (2009): pp.1163–69.
- M. Cohen et al., “Prevention of HIV-1 infection with early antiretroviral therapy,” New England Journal of Medicine 365/6 (2011): pp.493–505; A. Anglemyer et al., “Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples,” Cochrane Reviews, 4 (2013).
- S. Biraro et al., “HIV-1 Transmission within marriage in rural Uganda,” PloS One 8/2 (2013).
- F. Tanser et al., “High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal,” Science 339/6122 (2013): pp.966–71.
- B. Williams et al., “Antiretroviral therapy for tuberculosis control in nine African countries,” Proceedings of the National Academy of Sciences 107/45 (2010): pp.19485–89.
- J. Bor et al., “Increases in adult life expectancy in rural South Africa: Valuing the scale-up of HIV treatment,” Science 339/6122 (2013): pp.961–65.
- R. Walensky et al., “Cost-effectiveness of HIV treatment as prevention in serodiscordant couples,” New England Journal of Medicine 369/18 (2013): pp.1715–25; J. Sempa et al., “Cost-effectiveness of early initiation of first-line combination antiretroviral therapy in Uganda,” BMC Public Health 12/1 (2012): p.736; R. Granich et al., “Expanding ART for treatment and prevention of HIV in South Africa,” PLoS One 7/2 (2012): p.e30216.
- H. Thirumurthy et al., “HIV treatment produces economic returns through increased work and education, and warrants continued US support,” Health Affairs 31/7 (2012): pp.1470–77.
- A. Margolis et al., “A review of the toxicity of HIV medications,” Journal of Medical Toxicology 10/1 (2014): pp.26–39.
- P. Clayden et al., 2013 Pipeline report: HIV, Hepatitis C, and Tuberculosis drugs, diagnostics, vaccines, preventive technologies, research toward a cure, and immune-based and gene therapIes in development (TAG, 2013).
- C. Emdin, N. Chong, and P. Millson, “Non-physician clinician provided HIV treatment results in equivalent outcomes as physician-provided care,” Journal of the International AIDS Society 16/1 (2013): p.18445; N. Mdege, S. Chindove, and S. Ali, “The effectiveness and cost implications of task-shifting in the delivery of antiretroviral therapy to HIV-infected patients,” Health Policy & Planning 28/3 (2013): pp.223-36
- J. Mann et al., “Health and human rights,” Health and Human Rights, 1/1 (1994): pp.6-23; R. A. Smith and P. D. Siplon, Drugs into bodies: Global AIDS treatment activism (Westport, CT: Praeger Publishers, 2006); M. Merson et al., “The history and challenge of HIV prevention,” The Lancet 372/9637 (2008): pp. 475–88.
- UN Human Rights Council, Report: Human Rights Council holds panel discussion on giving a voice to People Living HIV/AIDS (Geneva, 2012).
- D. Brock and D. Wikler, “Ethical challenges in long-term funding for HIV/AIDS,” Health Affairs 28/6 (2009): pp.1666–76.
- B. Meier, K. Brugh and Y. Halima, “Conceptualizing a human right to prevention in global HIV/AIDS policy,” Public Health Ethics 5/3 (2012): pp.263-282.
- S. Rennie and F. Behets, “Desperately seeking targets,” Bulletin of the WHO 84/1 (2006): pp.52–57; GNP+ et al., Access challenges for HIV treatment, (Geneva: GNP, 2013), http://www.gnpplus.net/assets/Access_Challenges_for_HIV_treatment_among_PLHIV_and_KAPs_in_MICs_Policy_brief-copy.pdf.
- L. Jones et al., “Costing human rights and community support interventions as a part of universal access to HIV treatment and care in a Southern African setting,” Current HIV Research 9/6 (2011): p.416.
- E. Kinney, “International human right to health: What does this mean for our nation and world,” Indiana Law Review 34 (2000): p.1457.
- UNCESCR, General Comment No. 14. E/.12/2000/4. (2000). Available at http://www.unhchr.ch/tbs/doc.nsf/%28symbol%29/E.C.12.2000.4.En; UNCESCR, General Comment No. 3. E/1991/23. (1990).
- J. Heymann et al., “Constitutional rights to health, public health and medical care: The status of health protections in 191 countries,” Global Public Health 8 (2013): pp.639–53.
- J. Biehl et al., “Judicialisation of the right to health in brazil,” The Lancet 373/9682 (2009): pp. 2182–84; B. Meier and A. Yamin, “Right to health litigation and HIV/AIDS policy,” Journal of Law, Medicine & Ethics 39 (2011): pp.81-84; A. Yamin and S. Gloppen, Litigating health rights (Cambridge: Harvard, 2011); O. Norheim and B. Wilson, “Health rights litigation and access to medicines,” Health and Human Rights 16/2 (2014); V. Gauri and D. Brinks, Courting social justice (Cambridge: Cambridge University Press, 2008).
- Minister of Health and others v. TAG and Others, 721, 34 (Constitutional Court 2002); R. Goldstone, “A South African perspective on social and economic rights,” Human Rights Brief 13 (2006), pp. 4–74.
- K. Young, “The minimum core of economic and social rights,” Yale International Law Journal 33 (2008); L. Gostin et al., “The joint action and learning initiative on national and global responsibilities for health,” World Health Report 2010, pp. 1261–74; P. Hunt and G. Backman, “Health systems and the right to the highest attainable standard of health,” Health and Human Rights 10/1 (2008), pp. 81–92; L. Forman et al., “What could a strengthened right to health bring to the Post-2015 Health Development Agenda,” BMC International Health and Human Rights 13/1 (2013), p. 48.
- World Health Organization, Consolidated guidelines on the use of antiretroviral drugs for treating and treventing HIV infection. (Geneva: WHO, 2013).
- UNCESCR, General Comment No. 14. E/.12/2000/4. (2000). Available at http://www.unhchr.ch/tbs/doc.nsf/%28symbol%29/E.C.12.2000.4.En.
- Guidelines for the Use of Antiretroviral Agents (Washington: Department of Health & Human Services, 2013). Available at: aidsinfo.nih.gov/guidelines; R. Granich and S. Gupta, The Global Database of National HIV/AIDS Guidelines (Geneva: UNAIDS (Personal Communication), 2015).
- WHO (See note 35, 2013).
- UNAIDS, Report on the Global AIDS Epidemic 2013 (Geneva: UNAIDS, 2013).
- WHO, Global Update on HIV Treatment 2013 (Geneva: WHO, 2013), p.11, Available at http://www.who.int/hiv/pub/progressreports/update2013/en/index.html.
- G. Backman et al., “Health systems and the right to health,” The Lancet 372/ 9655 (2008), pp. 2047–2085.
- C. Mugglin et al., “Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub Saharan Africa: Systematic review and meta analysis,” Tropical Medicine & International Health 17,/12 (2012), pp. 1509–1520.
- M. Callaghan, N. Ford, and H. Schneider, “A systematic review of task-shifting for HIV treatment and care in Africa,” Human Resources for Health 8/8 (2010), pp. 8–16.
- J. Stockman, M. Lucea, and J. Campbell, “Forced sexual initiation, sexual intimate partner violence and HIV risk in women,” AIDS and Behavior 17/3 (2013), pp.832–47; T. Rhodes et al., “Structural violence and structural vulnerability within the risk environment,” in Rethinking social epidemiology (NY: Springer, 2012), pp. 205–30; T. Holtz et al., “Longitudinal analysis of key HIV-risk behavior patterns and predictors in MSM,” Archives of Sexual Behavior 44/2 (2015): pp. 341–48.
- A. Yamin and R. Cantor, “Between insurrectional discourse and operational guidance,” Journal of Human Rights Practice 6/3 (2014), pp. 451–85.
- UNAIDS (See note 39, 2013); UNAIDS, Meeting the investment challenge (Geneva: UNAIDS, 2012).
- UNAIDS/AU, Abuja +12 (2013), Available at http://www.unaids.org/en/2fsites/2fdefault/2ffiles/2fmedia_asset/2fjc2524_abuja_report_en_0.pdf
- CEHURD & Ors v. Attorney General, Uganda Civil suit No.111 (2012).
- Gostin et al., “The joint action and learning initiative on national and global responsibilities for health” (See note 34).
- 2001 Declaration of Commitment on HIV/AIDS (New York, 2001); Declaration on the TRIPS Agreement and Public Health, World Trade Organization: WT/MIN(01)/DEC/2, 2001.
- J. Kates, A. Wexler, and E. Lief, Financing the response to HIV in low- and middle-income countries (Washington, DC: KFF, September 2013).
- UNAIDS, Fast-track (Geneva: UNAIDS, 2014).
- Clinton Foundation, “Study finds cost of treating HIV patients is far lower than commonly believed,” 2012, Available at: https://www.clintonfoundation.org/main; G. Jouquet et al., “Economic evaluation and financing HIV/AIDS: Cost analysis of an ARV cure,” in 5th International AIDS Society Conference, 2009, 19–22.
- Bown v. Kendrick, 487 U.S. 589 (USSC 1988); American Academy of Pediatrics v. Clovis Unified School District, 8 (Superior Court of the State of California, County of Fresno 2012).
- J. E. Stiglitz, “Economic foundations of intellectual property rights,” Duke Law Journal 57 (2007): p.1693–724; K. Maskus, Private Rights and Public Problems: The Global Economics of Intellectual Property in the 21st Century (Peterson Institute, 2010); B. Baker, “Debunking IP-For-Development: Africa needs IP space not IP shackles,” in International economic law and African development (Johannesburg: Siber, 2014).
- P. Cawthorne et al., “Access to drugs: The case of Abbott in Thailand,” The Lancet Infectious Diseases 7 (2007), pp. 373–74; E. t Hoen et al., “TRIPS, Pharmaceutical Patents and Access to Essential Medicines: Seattle, Doha and beyond,” in Economics of AIDS and Access to HIV/AIDS Care in Developing Countries. (Paris: ANRS, 2003).
- C. Correa and D. Matthews, The Doha Declaration 10 Years on and Its Impact on Access to Medicines and the Right to Health, (NY: UNDP, 2011), Available at: http://www.undp.org/content/dam/undp/library/hivaids/Discussion_Paper_Doha_Declaration_Public_Health.pdf.
- Global Commission on HIV and the Law, Report, July 2012.
- G. Ooms et al., “The ‘diagonal’ approach to global fund financing: A cure for the broader malaise of health systems,” Global Health 4/6 (2008).
- B. Samb et al., “An assessment of interactions between global health initiatives and country health systems,” The Lancet 373/9681 (2009), pp. 2137–69.
- United States Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. (accessed May 2015)
- Barnhart and Shelton, “ARVs: The next generation. Going boldly together to new frontiers of HIV Treatment,” Global Health, 2015.
- S. Epstein, “Activism, drug regulation, and the politics of therapeutic evaluation in the AIDS Era,” Social Studies of Science 27/5 (1997): pp.691–726.
- M. Bemelmans et al., “Community‐supported models of care for people on HIV treatment in sub‐Saharan Africa,” Tropical Medicine & International Health 19/8 (2014): 968–77; WHO, Supplement to the 2013 Consolidated Guidelines (Geneva: WHO, 2014).
- J. Prah Ruger, “Health capability,” American Journal of Public Health 100/1 (2010): p.41.
- D. Barr, J. Amon, and M. Clayton, “Articulating a rights-based approach to HIV treatment and prevention interventions,” Current HIV Research 9/6 (2011): pp.396–404.
