Gitau Mburu, Enrique Restoy, Evaline Kibuchi, Paula Holland, and Anthony D. Harries
Abstract
Adherence to treatment is a key element for global TB control. Public health laws can be used to enforce isolation, adherence, and completion of TB treatment. However, the practical application of public health laws can potentially range from voluntary measures to involuntary detention approaches. This paper explores the potential risks and impacts of using detention approaches to enforce TB treatment adherence. In August 2015, we conducted a literature search regarding the application of public health laws to enforce adherence to TB treatment globally, and specifically in Kenya. Texts were analyzed using narrative synthesis. Results indicated that in Kenya, people lost to follow-up on TB treatment were frequently detained in prisons. However, incarceration and detention approaches curtail the rights to health, informed consent, privacy, freedom from non-consensual treatment, freedom from inhumane and degrading treatment, and freedom of movement of people lost to follow-up. Detention could also worsen social inequalities and lead to a paradoxical increase in TB incidence. We suggest the incorporation of less intrusive solutions in legislation and policies. These include strengthening health systems to reduce dependency on prisons as isolation spaces, decentralizing TB treatment to communities, enhancing treatment education, revising the public health laws, and addressing socioeconomic and structural determinants associated with TB incidence and loss to follow-up.
Introduction
The World Health Organization (WHO) estimates that 9 million people develop tuberculosis (TB) annually, a sixth of whom die as a result.1 In 2014, this translated to 1.6 million deaths, of which more than 90% were in developing countries.2 The clustering of TB in low- and middle-income countries is not surprising. An increasing amount of evidence suggests that individual vulnerability to TB is determined by risk factors that are often related to a person’s social and economic position. This association has led some commentators to label TB as “traditionally a disease of the poor.”3 TB is associated with being malnourished, smoking, alcohol abuse, exposure to indoor air pollution, and living or working in crowded and poorly ventilated conditions.4 TB prevalence is also high among individuals confined in prisons.5 There is considerable evidence that people in lower socioeconomic groups are, on average, more likely to possess these risk factors or determinants, including those living in developed countries.6 Consequently, although context-specific differences may exist, overall, TB is more common in developing countries, where poverty, poor housing conditions, and indoor air pollution are more frequent, and expenditure on health is low.7 Over 80% of TB cases and deaths occur in low- and middle-income countries, and 80% of the global burden of TB is concentrated in just 22 countries, of which 20 are Asian or African.8
Kenya is among the 22 countries with the highest burden of TB, and in 2014, there were an estimated 110,000 TB cases.9 The prevalence of multidrug-resistant TB (MDR-TB: tuberculosis that is resistant to the two main first-line anti-tuberculosis drugs isoniazid and rifampicin) has increased in Kenya over the last few years, from 0.04% in 2005 to 0.16% in 2011—a four-fold increase in six years, partly because of poor adherence.10 Evidence suggests that MDR-TB is particularly common in deprived neighborhoods. Two studies conducted between 2011 and 2012 showed that the level of MDR-TB in Nairobi’s Kibera slums was typically between 0.5% and 1% among new TB cases and 8.5% among recurrent TB cases.11 Because MDR-TB is difficult and more expensive to treat, its case fatality rate is almost twice that of drug-susceptible TB.12 Given the increased risk of transmission of pulmonary TB, treatment completion and infection control are emphasized.13
Although of public health importance, adherence to a full course of TB treatment is not always easy. A full course of treatment involves a combination of four drugs taken orally, daily, for two months, followed by two drugs taken orally for four months.14 A recent systematic review demonstrated a range of factors that contribute to poor adherence to TB drugs globally, including poor availability and organization of health services, local interpretations of illness, financial burdens around accessing treatment, poor knowledge or negative attitudes about TB, side effects of TB drugs, and lack of family support.15 This contextualization is central to our understanding of the impact of incarceration and compulsory detention approaches of enforcing adherence to TB treatment, which is the focus of this paper.
In general terms, combinations of downstream and upstream interventions are used to facilitate adherence. An example of a downstream strategy is the WHO DOTS strategy (Directly Observed Treatment, Short Course) which was conceived in 1994 specifically to support detection and successful treatment of TB.16 One component of the DOTS strategy is direct observation of treatment, either by a health care provider or family member. One of the targets of the DOTS strategy is to achieve 85% treatment success, that is, 85% of TB patients complete their treatment and are declared no longer infectious.17 This target has been achieved in many countries. For example, in 2011, the treatment success among 82,000 people on TB therapy in Kenya was 86%.18 However, as we argue in this paper, this target has partly been achieved through a process involving involuntary enforcement of adherence, specifically through incarceration, raising concerns regarding the appropriateness of these approaches to achieve treatment objectives.
Although an ongoing challenge is to ensure that all people with TB (estimated to be about 110,000 cases per year in Kenya) are detected and started on treatment, mandatory isolation and its impact on individuals with TB, as well as overall prevalence rates, needs to be better understood.19 Mandatory isolation is an example of an upstream intervention applied for the purposes of infectious disease containment, including TB. This “typically involves detection, notification, quarantine, and isolation of actual or suspected cases, the protection and monitoring of those not infected, and possibly even treatment” for those infected.20 In many countries, public health laws provide powers to enforce isolation and compulsory treatment of TB.21 However, the extent of the application of this strategy and its potential impact on individual rights is poorly documented. The aim of this paper is therefore to describe the potential risks of incarceration and compulsory detention as a means of enforcing adherence to TB treatment, and to suggest human rights-based alternatives to this approach, using Kenya as an example.
Methods
In August 2015, we conducted a literature search regarding the application of public health laws as an instrument for enforcing adherence to TB treatment globally, and specifically in Kenya. We searched Medline, PubMed, Social Policy and Practice, and Web of Science for peer-reviewed articles, and searched websites of global health governance institutions such as WHO, the Global Fund to Fight AIDS, Tuberculosis and Malaria, PEPFAR/USAID, as well as Google Scholar, for grey literature. The search was performed using a combination of terms relating to TB (‘TB’, ‘tuberculosis’), public health laws (‘law’, ‘public health act’, ‘control act’, ‘disease act’ and ‘health law’), and adherence (‘compliance’, ‘default’, ‘loss to follow-up’, and ‘treatment completion’). Truncation, Boolean, and proximity operators were used when appropriate to increase sensitivity of the search. Two authors (GM and ER) identified relevant articles, focusing on human rights aspects of public health responses to TB and other communicable diseases. Analysis of the results was conducted using narrative synthesis in which relevant information is collated to inform practice and policy.22 In our analysis, we aimed to compare the practice and application of the Public Health Act in Kenya with that described in other countries, using recent reports of how the Act has been used in Kenya as a starting point. We did not perform in-depth scrutiny of the Public Health Act itself, apart from its application.
Results
International human rights standards and public health laws
We identified evidence of the existence of public health laws for the purpose of controlling infectious diseases in different contexts, including in Europe, Asia, Middle East, North America, and South Africa, among others.23 Public health laws are widely applied to facilitate international containment of infectious diseases and achieve health protection, for instance, through TB screening at international border points.24 Furthermore, a variety of more specific laws related to infectious disease are used for controlling the spread of a wide variety of notifiable diseases including and beyond TB.25 Evidence suggests that legislative control of TB transmission has recently regained attention due to the threat of multidrug-resistant TB.26 A recent survey of 14 European countries identified, mapped, analyzed, and described legislative tools used to support tuberculosis control.27 It found that a wide range of compulsory legal measures such as examination, screening, detention, treatment, and vaccination were available as ways of protecting the public health.28 More specifically, use of compulsory isolation of TB patients appears to be commonly provided by the law in different countries, with a number of high-burden countries having current legislation allowing for this practice, including Armenia, Belarus, Georgia, the Republic of Moldova, and Ukraine.29 In other high-burden countries such as South Africa, proposals for detention have been opposed.30 In most countries, literature referred to isolation wards or hospitals.31 However, prison-based isolation appears to be rare, with cases reported in Israel and Kenya, although the facilities in prison differed.32 In Israel, patients who are lost to follow-up on treatment were either hospitalized under a court order and after failing to comply with the order, hospitalized in prison or referred directly to a prison hospital.33 In Kenya, people lost to follow-up were detained in ordinary prisons without health facilities.34
Despite these legal provisions of compulsory isolation, human rights literature suggests that compulsory treatment infringes on the right to health of people lost to follow-up in at least two ways. First, this practice generally disregards evidence-based medicine, therefore failing to meet the quality requirement of the right to health. Second, this practice disregards informed consent, which is a critical element of voluntary counseling, testing, and treatment.35 However, the right to health of other members of society cannot be ignored and the state has a responsibility to protect the public from unnecessary risks of contracting airborne diseases and thus must balance involuntary confinement of people lost to follow-up and public health protection of the wider population.36
The Siracusa Principles, which were adopted by the UN Economic and Social Council, allow for the limitation of individual rights as a means to deal with “a serious threat to the health of the population or individual members of the populations.”37 The Siracusa Principles specify that the limitations of individual rights may only occur when such limitations are: (1) provided for and carried out in accordance with the law; (2) directed towards a legitimate objective of general interest; (3) strictly necessary in a democratic society; (4) the least intrusive and restrictive in severity and duration to achieve the objective; and (5) based on scientific evidence and neither drafted nor imposed arbitrarily nor in a discriminatory manner.38 In this context, WHO suggests that interfering with freedom of movement when instituting quarantine or isolation for a communicable disease such as drug-resistant TB could be legitimate under international human rights law. However, WHO stresses that “this must be viewed as a last resort, and justified only after all voluntary measures to isolate such a patient have failed.”39 Most recently, for example, individual rights and freedoms were curtailed for a limited time within these provisions during the Ebola outbreak.40
Current application of the Public Health Act as an instrument for enforcing treatment adherence in Kenya
The Public Health Act constitutes Chapter 242 of the Laws of Kenya. It has existed since September 6, 1921, and has been applied as a tool for TB containment.41 Specifically, Section 27 of the Act makes provisions for the mandatory isolation of a person with an infectious disease. It states that:
Where, in the opinion of the medical officer of health, any person has recently been exposed to the infection, and may be in the incubation stage of, or is infectious with any notifiable infectious disease and is not accommodated in such manner as adequately to guard against the spread of the disease, such person may, on a certificate signed by the medical officer of health, be removed, by order of a magistrate at the cost of the local authority of the district where such person is found, to a place of isolation and there detained until, in the opinion of the medical officer of health, he/she is free from infection or able to be discharged without danger to the public health, or until the magistrate cancels the order.42
In relation to TB, this includes people lost to follow-up on treatment. Because the health system is relatively under-resourced, proper isolation facilities in hospitals hardly exist at the district level, and people lost to follow-up are instead frequently detained in prison where they are compelled to take treatment until they complete the full course.43 Literature suggests that detention in prison is often implemented in Kenya, with documented cases in 2010, 2011, and 2015.44
Impact and appropriateness of prison-based mandatory custodial isolation in Kenya
The legal soundness of the compulsory measures described in the previous section can be assessed against the conditions provided in the Siracusa Principles, as well as guidance provided by WHO and other key actors of the global health governance involved in the response to TB.45 As described earlier, the Siracusa Principles only allow for the limitation of individual rights as a last resort when voluntary measures, on which public health should be based, may be ineffective.46 These limitations must be substantiated by scientific evidence.47
The present application of incarceration in Kenya raises concerns both in terms of human rights law and scientific evidence, thus questioning whether application of the Siracusa Principles is appropriate and calling for the consideration of human rights-based alternatives, which may also be effective from a public health point of view.
Firstly, in relation to international human rights law, compulsory isolation in prison curtails human rights as it curtails the right to voluntary informed consent, an integral part of the enjoyment of the right to health as enshrined in numerous international and national human rights instruments and legislation.48 Article 12 of the International Covenant on Economic, Social, and Cultural Rights (ICESCR) addresses the right to the highest attainable standard of physical and mental health, which includes the right to be free from non-consensual medical treatment.49 Incarceration also contravenes the freedom of movement of people lost to follow-up and could also breach their rights to privacy.50 Such detention may cause further spread or reinfection with TB when people lost to follow-up are held in prisons, which are typically overcrowded and poorly ventilated, denying the right to health of fellow prisoners, in addition to those lost to follow-up.51 This situation also denies access to health facilities and access to goods and services that are scientifically appropriate and of good quality.52 In this context, the limitations of the right to freedom of movement of people lost to follow-up are not justified under the Siracusa Principles, since they fail to contribute to effective TB control and sound public health response. This is particularly relevant given that a study among prisoners in Kenya showed that most prisoners were exposed to, and subsequently acquired, TB in prison, not outside of it.54
Key actors in global health governance, such as the Global Fund, encourage human rights responses to TB and explicitly acknowledge that “mandatory treatment or confinement” could be contributing to a rise rather than a reduction of drug-resistant TB, therefore undermining the right to health for both fellow prisoners without TB and the larger population.54 Indeed, WHO asserts that in most cases detention approaches are not justified, because other less intrusive and less restrictive measures have not been exhausted before, as recommended by Siracusa Principles.55 This is particularly relevant considering that the application of detention measures is rarely proportionate to the risk of transmission posed by those who are lost to follow up. WHO states that by the time a diagnosis is made, household contacts are already exposed to TB and the probability of airborne transmission goes down markedly after treatment is started.56 Most of the people who are incarcerated will have started treatment but failed to adhere to it.57 Taken together, these concerns indicate that incarceration is a disproportionate response to the failure to adhere to TB treatment.
Secondly, global public health is progressively moving away from authoritarian control paradigms toward empowering individuals to take greater control of their health, as emphasized in the 1979 Alma-Ata Declaration.58 Fidler et al. argue that quarantine and isolation are society’s self-preservative responses to the threat of contagious diseases.59 However, as Zachariah et al. contest, an authoritarian paradigm is particularly strong in TB programs as exemplified by the popular use of terms such as ‘TB control,’ ‘TB suspect,’ and ‘defaulter.’ They argue that this is linked to a “paternalistic public health approach that makes the public health official the decision-maker on behalf of the patient.”60 In summary, the current approach of mandatory custodial isolation disempowers communities and is contrary to patient-centered care. Akugizibwe and Ramakant further assert that subjecting patients to punitive public health interventions, which they argue is anchored on the historically coercive model of TB treatment, could lead to violation of patients’ rights and reinforce TB stigma.61 These observations have partly led the Stop TB Partnership to issue global guidance calling for a shift towards patient-centered language in TB communication.62
Thirdly, mandatory custodial isolation does not account for the wider epidemiological and health systems context in Kenya, especially the ability of the health system to support its application as intended. This consideration is important because in many countries, control of TB is hampered by “restricted capacity within overburdened health systems.”63 We contend that in the Kenyan context, where 6-7% of an estimated 110,000 people with TB are lost each year to follow-up, this policy may not be effectively operationalized, because health systems are insufficiently resourced in terms of proper isolation wards and human resource capacity.64 Based on our findings, the impact of health systems’ capacity on the decentralized implementation of mandatory isolation appears not to have been evaluated to date. Literature clearly suggests that although some countries have legislation permitting involuntary isolation of TB patients, some of these countries are not applying the legislation as they consider that they lack the necessary resources to provide adequate isolation health facilities. Such is the case with Armenia and parts of Ukraine.65 Arguably, people lost to follow-up on TB treatment in Kenya are, at least in part, punished due to the failings of the health systems.
Fourthly, mandatory custodial isolation could reinforce existing inequalities through the socioeconomic deprivation of people with TB, thereby increasing their vulnerability on the grounds of their social and economic status instead of protecting them as required by international law.66 Isolation for prolonged periods can lead to loss of livelihoods for imprisoned people and their families.67 Lack of employment itself has been independently associated with TB among prisoners in Kenya.68 Loss of income for incarcerated patients could drive their dependent families to cheaper, smaller, and potentially overcrowded or poorly ventilated housing, further increasing their vulnerability to TB. A recent study among a general cohort of TB patients from a Kenyan slum found that low income was a predictor of defaulting treatment.69
Given that TB occurs mostly among poor populations, this can create the potential for a vicious cycle and social patterning of TB that result in isolation, loss of livelihoods and employment, relocation to cheaper housing, recurrence of TB, and further loss to follow-up and subsequent incarceration. Owing to the underlying population clustering of economically disadvantaged social groups in deprived slums, this has led to what Kawachi et al. refer to as ‘spatial disease patterns’—where slums have differentially high rates of TB in Kenya, including multidrug-resistant TB.70 It is not surprising that among urban poor in Kenya, previous loss to follow-up is a strong predictor of future loss to follow-up.71
Finally, mandatory isolation of people lost to follow-up could adversely affect their psychological well-being, thus undermining their right to achieve their highest attainable standard of mental health.72 As Zachariah et al. and Akugizibwe and Ramakant argue, punitive measures could inadvertently result in people lost to follow-up being treated like criminals.73 Considering that in Kenya, mandatory isolation takes place in prisons, and thus people lost to follow up are imprisoned like other inmates, this approach equates to criminalization of poor compliance with treatment.74 This disproportionately punishes people lost to follow-up, and their families and dependents, fueling the perception that they are criminals requiring disciplinary treatment approaches, and may undermine health-promotion initiatives, perpetuate stigma, and increase health risks of the wider populations.75
The resulting stress and social exclusion associated with criminalization could lead to substance abuse, poor health outcomes, and an exacerbation of health inequalities through psychosocial pathways.76 A study conducted in Kenya in 2011 showed that the odds of a person lost to follow-up on TB treatment being an alcohol abuser were up to five times that of someone adhering to treatment.77 In addition, coercive measures can reinforce stigma, which is an important determinant of treatment completion in Kenya.78 In summary, incarceration and detention approaches are likely to exacerbate pre-existing social inequities.
Human rights-based alternatives
The suitability of detention to the Kenyan context remains contentious and there are numerous concerns regarding its impact on both individual human rights and collective public health interests. We suggest that potential alternative solutions fall into three broad categories: preventing primary loss to follow-up, improving premises and conditions of isolation, and amending public health laws to exclude prison as a setting for mandatory isolation. The combination of these measures aims to offer alternatives to current detention approaches that infringe on the rights of people lost to follow-up without compromising public health TB control.
Primary prevention of loss to follow-up
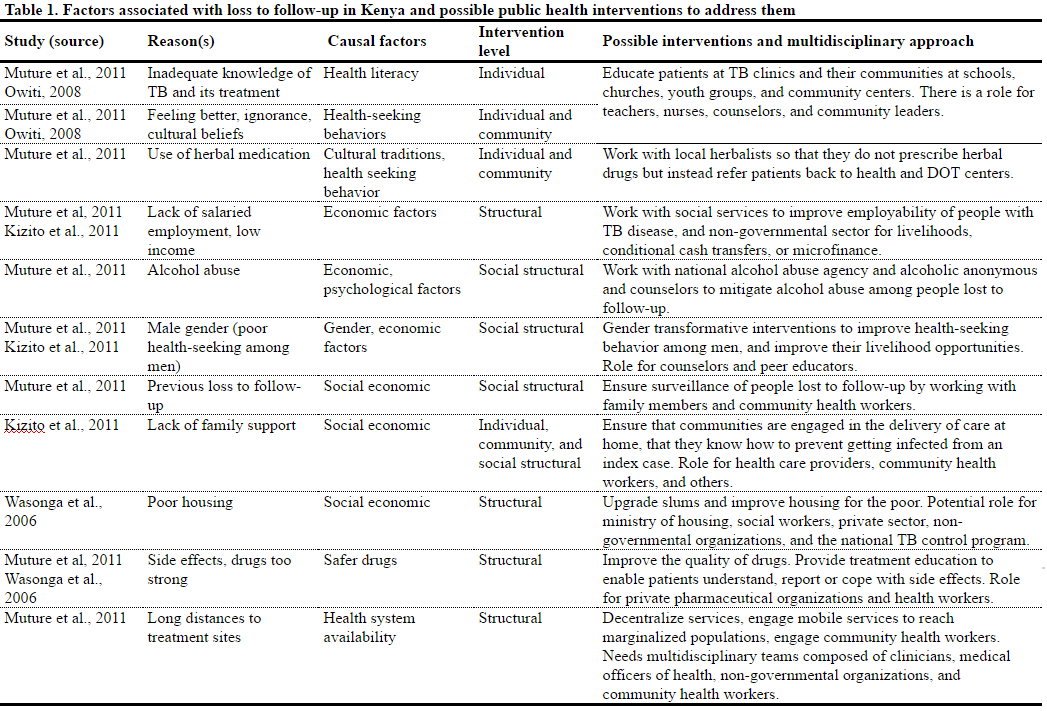
Understanding why people get lost to follow-up is central to our proposed potential solutions. The reasons why people stop TB treatment, and how they can best be supported to adhere and complete full course of treatment need to be understood from an individual, household, and community perspective.79 Studies suggest that common reasons why people on treatment for TB are lost to follow-up include lack of adequate knowledge about TB, lack of adequate family support, cultural beliefs which curtail health seeking, and living a long distance from health facilities (Table 1).80 The WHO asserts that non-adherence is often a direct consequence of failure to educate and engage people with TB in the treatment process, which is common in the TB sector.81 Hence, counseling people with TB, strengthening treatment literacy, and respectful engagement of TB patients to enable them to make informed decisions regarding treatment could potentially reduce rates of loss to follow-up. In addition, such patient-centered strategies are consistent with a human rights approach as they promote access to essential physical, informational, and economic elements of the right to health.82
Although it is not the intention of this paper to comprehensively review all causal reasons, our view is that socioeconomic factors, such as poverty, lack of employment, lack of transport, and poor housing, are central to both the acquisition of TB and loss to follow-up, and that these factors are not easily amenable to vertical interventions such as incarceration. A systematic review conducted by Munro et al. identified similar social and structural factors as key drivers of poor adherence globally.83
In Kenya, there is a significant policy focus on health promotion and education, tracing persons lost to follow-up, and enforcing treatment completion.84 However, there is little focus on countering some of the social-structural reasons for stopping TB treatment, such as long distances to health facilities, poverty, gender, and alcohol abuse, as part of TB interventions.85 A fundamental argument against focusing on medical, educational, or legal interventions such as incarceration is that doing so does not address important structural drivers of stopping TB treatment.86 Therefore, to prevent primary loss to follow-up, structural interventions are also required, and implementing these may require multi-sectoral partnerships beyond the remit of a typical national TB program.87 In addition, addressing the social determinants of adhering to TB treatment could potentially reduce both the incidence of TB and loss to follow-up.
Alternatives to detention-based approaches
The second solution is focused on finding alternatives to the ongoing use of prisons as spaces for isolation. This could be achieved by strengthening health systems to increase the capacity of hospitals, decentralizing provision of TB medications deep into communities, and providing functional isolation wards and hospitals. Hence, isolation could be conducted within isolation health facilities appropriate for that purpose. In addition, evidence suggests that community-based adherence support can be a viable alternative; WHO supports a community-based treatment approach.88 The primary aim of alternatives to incarceration is to achieve infection control to prevent spread of TB, and at the same time respect the dignity and human rights of those lost to follow-up, ensuring that they are free from stigma and dehumanizing treatment and, where possible, enjoy freedom of movement.89
Revision of the Public Health Act and formulation of new isolation policy
The third human rights-based solution we propose is to amend the Public Health Act to clearly indicate that prisons should not be used for enforcing treatment. The ground for this has recently been laid. Pursuant to petition 329 of 2014 in the High Court of Kenya at Nairobi, a determination was made—while our research was in progress—making it unconstitutional to confine persons with TB in prison for the purpose of treatment on the basis of the Public Health Act.90 In addition, the court ordered appropriate policies for the confinement of persons with TB and other infectious diseases to be formulated by the Ministry of Public Health. The text of the Public Health Act therefore needs to be amended to be consistent with the above judgment, given the reliance on the Act for public health protection.
Consequently, development of progressive policies is now required. The Ministry of Public Health should strengthen community-based support. The new policy could require health care workers to provide adherence support to lost to follow up TB patients in their homes, for example through periodic home visits, as is the case in other countries that have shifted from detention approaches.91 Community health workers, who have effectively supported adherence to HIV treatment, could be a suitable alternative for performing this role, given existing human resource capacity constraints.92 In addition, new policies should emphasise the importance of patient engagement in treatment decisions and the rights of TB patients.93
Given this paradigm shift, it is critical that the Ministry of Public Health provides training and resources to respect and protect these rights within health care facilities in the context of patient- centered care and infection control. Further, new policies should emphasize that even for non-adherent persons, isolation in proper health facilities should only be the last resort and even then, such patients could still choose not to take medications. In such a scenario, isolation could only be useful for public health protection for the limited duration of infectiousness, as emphasized by WHO.94 To be effective, a range of socio-economic and structural interventions highlighted in Table 1 should form a central part of new policies anchored on social support, as recommended by WHO.95
Conclusions
We have presented a range of less restrictive and human rights-based alternatives to incarceration and detention-based approaches as a means of enforcing involuntary treatment of TB. We have illustrated ways in which such incarceration and detention-based approaches are contrary to international human rights provisions, and indeed, how they could worsen social inequalities and lead to paradoxical outcomes in regard to TB. In light of the recent case in the High Court of Kenya at Nairobi forbidding use of prisons as a place of mandatory isolation for people who have stopped their TB treatment, we suggest that there is a need to strengthen the health systems to reduce dependency on prisons as isolation spaces by creating proper isolation facilities, wards, and hospitals, further decentralize services to communities, and revise the Public Health Act and formulate a new isolation policy. In addition, collaboration with other sectors to address socio-economic and structural determinants of TB infection and loss to follow-up is required.
Gitau Mburu, MD, DTM&H, MPH, is a Senior Advisor for HIV and Health Systems at the International HIV/AIDS Alliance, Brighton, United Kingdom.
Enrique Restoy, MA, PhD, is Senior Human Rights Advisor at the International HIV/AIDS Alliance, Brighton, United Kingdom.
Evaline Kibuchi, MA, PGD, is Chief National Coordinator of the Stop TB Partnership – Kenya.
Paula Holland, MA(Econ), PhD, is a Lecturer in Public Health in the Division of Health Research at Lancaster University, United Kingdom.
Anthony D. Harries, OBE, MA, MD, FRCP, DTM&H, is Senior Advisor and Director of the Department of Research at the International Union Against Tuberculosis and Lung Disease, Paris, France, and an honorary professor at the London School of Hygiene and Tropical Medicine, London, United Kingdom.
Please address correspondence to the authors c/o Gitau Mburu, International HIV/AIDS Alliance, Preece House, 91-101 Davigdor Road, Hove, East Sussex, BN3 1RE, UK. Email: gmburu@aidsalliance.org.
Competing interests: None declared.
Copyright © 2016 Mburu, Restoy, Kibuchi, Holland, and Harries. This is an open access article distributed under the terms of the Creative Commons Attribution Non- Commercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
1. World Health Organization, 2015 Global tuberculosis report (Geneva: WHO, 2015).
2. Ibid.
3. D.P. Spence, J. Hotchkiss, C.S. Williams, et al., “Tuberculosis and poverty,” British Medical Journal 307/6907 (1993), pp. 759-61.
4. M.L. Hood, “A narrative review of recent progress in understanding the relationship between tuberculosis and protein energy malnutrition,” European Journal of Clinical Nutrition 67/11 (2013), pp: 1122-8; M.N. Bates, A. Khalakdina, M. Pai, et al., “Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis,” Archives of Internal Medicine 167/4 (2007), pp. 335-42; K. Lönnroth, B.G. Williams, S. Stadlin, et al., “Alcohol use as a risk factor for tuberculosis – a systematic review,” BMC Public Health 8 (2008), p. 289; K. Lönnroth, E. Jaramillo, B.G. Williams, et al., “Drivers of tuberculosis epidemics: the role of risk factors and social determinants,” Social Science and Medicine 68/12 (2009), pp. 2240-2246.
5. D. Pedrazzoli, L. Anderson, M. Lalor, et al., Tuberculosis in the UK 2013 report (London: Public Health England, 2013).
6. Ibid.; C. Dye, K. Lönnroth, E. Jaramillo, et al., “Trends in tuberculosis incidence and their determinants in 134 countries,” Bulletin of the World Health Organization 87/9 (2009), pp. 683-91.
7. World Health Organization (see note 1); Ibid.
8. World Health Organization (see note 1).
9. World Health Organization (see note 1).
10. B.R. Kidenya, L.E. Webster, S. Behan, et al., “Epidemiology and genetic diversity of multidrug-resistant tuberculosis in East Africa,” Tuberculosis 94/1 (2013), pp. 1-7.
11. T.D. Ogaro, W. Githui, G. Kikuvi, et al., “Anti-tuberculosis drug resistance in Nairobi, Kenya,” African Journal of Health Science 20 (2012), pp. 21-27; P.W. Ndung’u, S. Kariuki, Z. Ng’ang’a, et al., “Resistance patterns of Mycobacterium tuberculosis isolates from pulmonary tuberculosis patients in Nairobi,” Journal of Infection in Developing Countries 6/1 (2012), pp. 33-39.
12. World Health Organization (see note 1); Kidenya (see note 10).
13. World Health Organization, Treatment of Tuberculosis: Guidelines (Geneva: World Health Organization, 2010).
14. Ibid.
15. S.A. Munro, S.A. Lewin, H.J. Smith, et al., “Patient adherence to tuberculosis treatment: a systematic review of qualitative research,” PLoS Medicine 4/7 (2007), p. e238.
16. World Health Organization (see note 13).
17. Ibid.
18. World Health Organization (see note 1).
19. World Health Organization, Global tuberculosis report 2013 (Geneva: World Health Organization, 2013).
20. S. Gainotti, N. Moran, C. Petrini, et al., “Ethical models underpinning responses to threats to public health: a comparison of approaches to communicable disease control in Europe,” Bioethics 22/9 (2008), pp. 466-476.
21. R.J. Coker, S. Mounier-Jack, and R. Martin, “Public health law and tuberculosis control in Europe,” Public Health 121/4 (2007), pp. 266-273.
22. C. Pope, N. Mays, and J. Popay, “How can we synthesize qualitative and quantitative evidence for healthcare policy-makers and managers?” Healthcare Management Forum 19/1 (2006), pp. 27-31.
23. Gainotti (see note 20); D. Weiler-Ravell, A. Leventhal, R.J. Coker, et al., “Compulsory detention of recalcitrant tuberculosis patients in the context of a new tuberculosis control programme in Israel,” Public Health 118/5 (2004), pp: 323-8; L. London, “Confinement for extensively drug-resistant tuberculosis: balancing protection of health systems, individual rights and the public’s health,” International Journal of Tuberculosis and Lung Disease 13/10 (2009), pp. 1200-1209; J.E. van Steenbergen and A. Timen, “[The control of infectious diseases in The Netherlands],” Nederlands Tijdschrift voor Geneeskunde 149/4 (2005), pp. 177-181.
24. R. Griffith, “Protecting health through public health law,” British Journal of Nursing 22/22 (2013), pp: 1324-5; M. Lavender, “Tuberculosis in the United Kingdom. System for screening new immigrants is inadequate,” British Medical Joural 312/7033 (1996), p. 776.
25. Steenbergen (see note 23).
26. R. Coker, M. Thomas, K. Lock, et al., “Detention and the evolving threat of tuberculosis: evidence, ethics, and law,” Journal of Law, Medicine, and Ethics 35/4 (2007), pp. 609-615; D.P. Fidler, L.O. Gostin, and H. Markel, “Through the quarantine looking glass: drug-resistant tuberculosis and public health governance, law, and ethics,” Journal of Law, Medicine, and Ethics 35/4 (2007) pp. 616-628.
27. Coker (see note 21).
28. Ibid.
29. World Health Organization, Tuberculosis, ethics and human rights. Report of a regional workshop (Copenhagen: World Health Organization, 2013).
30. E. Goemaere, N. Ford, D. Berman, et al., “XDR-TB in South Africa: detention is not the priority,” PLoS Medicine 4/4 (2007), p. e162.
31. Coker (see note 26); Fidler (see note 26); World Health Organization (see note 29).
32. Weiler-Ravell (see note 23); AIDS Law Project, Actions Undertaken with Regard to Kenyan TB patient case (AIDS Law Project, 2010).
33. Weiler-Ravell (see note 23).
34. AIDS Law Project (see note 32); C. Wangechi, “TB patient jailed for defaulting treatment, in Kellect,” Internews Kenya (April 16, 2011) Available at http://www.internewskenya.org/summaries.php?id=915; C. Ngeno “Narok TB patient gets one year for failing to take medicine” The Standard (July 10, 2015) Available at http://www.standardmedia.co.ke/article/2000168622/narok-tb-patient-gets-one-year-for-failing-to-take-medicine.
35. United Nations, Special rapporteur on the right of everyone to the enjoyment of the highest attainable standard of physical and mental health. Report of the Special Rapporteur. (New York: United Nations, 2010); United Nations, Special rapporteur on the right of everyone to the enjoyment of the highest attainable standard of physical and mental health (New York: United Nations, 2009).
36. A. Boggio, M. Zignol, E. Jaramillo, et al., “Limitations on human rights: are they justifiable to reduce the burden of TB in the era of MDR- and XDR-TB?” Health and Human Rights 10/2 (2008), p. 121-6.
37. United Nations Commission on Human Rights, The Siracusa principles on the limitation and derogation provisions in the International Covenant on Civil and Political Rights (Geneva: United Nations Commission on Human Rights, 1984).
38. Ibid.; S. Abiola, The Siracusa Principles on the limitation and derogation provisions in the International Covenant for Civil and Political Rights (ICCPR): History and interpretation in public health context research memorandum prepared for the Open Society Institute’s Public Health Program Law and Health Initiative (2011).
39. World Health Organization, WHO guidance on human rights and involuntary detention for XDR-TB control (Geneva: World Health Organization, 2008).
40. D.S. Silva and M.J. Smith, “Limiting rights and freedoms in the context of Ebola and other public health emergencies: how the principle of reciprocity can enrich the application of the Siracusa Principles.” Health and Human Rights 17/1 (2015), pp. 52-57.
41. Laws of Kenya. Public Health Act Chapter 242 [1986] Revised 2012 (National Council for Law Reporting, 2012).
42. Ibid.
43. Division of Leprosy, Tuberculosis, and Lung Disease, Annual report 2012 (Nairobi, Kenya: Ministry of Health, 2012).
44. AIDS Law Project (see note 32); Wangechi (see note 34); Ngeno (see note 34).
45. Abiola (see note 38); World Health Organization (see note 39).
46. World Health Organization (see note 39); World Health Organization, Ethical considerations in developing a public health response to pandemic influenza (Geneva: World Health Organization, 2007).
47. United Nations (see note 35).
48. Ibid.
49. United Nations International covenant on economic, social and cultural rights, international covenant on civil and political rights and optional protocol to the international covenant on civil and political rights (New York: United Nations, 1966).
50. Boggio (see note 36).
51. S. Johnstone-Robertson, S.D. Lawn, Alex Welte, et al., “Tuberculosis in a South African prison – a transmission modelling analysis,” South African Medical Journal, 101/11 (2011), pp. 809-813; J. O’Grady, M. Hoelscher, R. Atun, et al., “Tuberculosis in prisons in sub-Saharan Africa–the need for improved health services, surveillance and control,” Tuberculosis 91/2 (2011), pp. 173-178.
52. United Nations Committee on Economic Social and Cultural Rights, The right to the highest attainable standard of health. Article 12, E/C. 12/2000/4, 2000. General Comment 14 (New York: United Nations, 2000).
53. A.S. Amwayi, G.M. Kikuvi, and E.M. Muchiri, “Modifiable factors associated with active pulmonary tuberculosis in a Kenyan prison,” East African Medical Journal, 87/2 (2010), pp. 43-48.
54. The Global Fund to Fight AIDS, Tuberculosis and Malaria, Tuberculosis and human rights information note (Geneva: The Global Fund to Fight AIDS, Tuberculosis and Malaria, 2013).
55. World Health Organization (see note 13); United Nations Commission on Human Rights (see note 37); Abiola (see note 38); World Health Organization (see note 39).
56. World Health Organization (see note 13); World Health Organization (see note 39).
57. AIDS Law Project (see note 32); Wangechi (see note 34); Ngeno (see note 34).
58. J.E. Lawn, J. Rohde, S. Rifkin, et al., “Alma-Ata 30 years on: Revolutionary, relevant, and time to revitalize,” Lancet 372/9642 (2008), pp: 917-27.
59. Fidler (see note 26).
60. R. Zachariah, A.D. Harries, S. Srinath, et al., “Language in tuberculosis services: Can we change to patient-centred terminology and stop the paradigm of blaming the patients?” International Journal of Tuberculosis and Lung Disease 16/6 (2012), pp. 714-717.
61. P. Akugizibwe and B. Ramakant, “Challenges for community role in tuberculosis response,” Lancet 375/9731 (2010), pp. 2059-2061.
62. Stop TB Partnership and UNOPS, Every word counts. Suggested language and usage for tuberculosis communications (Geneva: Stop TB Partnership, 2013).
63. R. Atun, D.E. Weil, M.T. Eang, et al., “Health-system strengthening and tuberculosis control,” Lancet 375/9732 (2010), pp. 2169-2178.
64. Kidenya (see note 10); Division of Leprosy, Tuberculosis, and Lung Disease (see note 43).
65. World Health Organization (see note 29).
66. United Nations Committee on Economic Social and Cultural Rights (see note 52).
67. R. Meek, “The possible selves of young fathers in prison,” Journal of Adolescence 34/5 (2011), pp. 941-949.
68. Amwayi (see note 53).
69. K.W. Kizito, S. Dunkely, M. Kingori, et al., “Lost to follow up from tuberculosis treatment in an urban informal settlement (Kibera), Nairobi, Kenya: What are the rates and determinants?” Transactions of the Royal Society of Tropical Medicine and Hygiene 105/1 (2011), pp. 52-57.
70. I. Kawachi, S.V. Subramanian, and N. Almeida-Filho, “A glossary for health inequalities,” Journal of Epidemiology and Community Health 56/9 (2002) pp. 647-652.
71. B.N. Muture, M.N. Keraka, P.K. Kimuu, et al., “Factors associated with default from treatment among tuberculosis patients in Nairobi province, Kenya: a case control study,” BMC Public Health 11 (2011), p. 696.
72. United Nations (see note 49).
73. Zachariah (see note 60); Akugizibwe (see note 61).
74. J.R. Hargreaves, D. Boccia, C.A. Evans, et al., “The social determinants of tuberculosis: From evidence to action,” American Journal of Public Health 101/4 (2011), pp. 654-662.
75. United Nations (see note 35, 2010)
76. Kawachi (see note 70); Krieger, N., “A glossary for social epidemiology,” Journal of Epidemiology and Community Health 55/10 (2001), pp. 693-700.
77. Muture (see note 71).
78. Akugizibwe (see note 61); I.W. Njau, S.M. Karanja, P. Wanzala, et al., “Factors associated with late presentation of suspected tuberculosis cases to tuberculosis management facilities: The case in Dagoretti district, Nairobi, Kenya,” Pan African Medical Journal 12 (2012), p. 93.
79. Commission on Social Determinants of Health, Closing the gap in a generation: Health equity through action on the social determinants of health (Geneva: WHO, 2008).
80. Kizito (see note 69); B. Nganda, J. Wang’ombe, K. Floyd, et al., “Cost and cost-effectiveness of increased community and primary care facility involvement in tuberculosis care in Machakos District, Kenya,” International Journal of Tuberculosis and Lung Disease 7/9 Suppl 1 (2003), pp: S14-20; D.M. Barter, S.O. Agboola, M.B. Murray, et al., “Tuberculosis and poverty: The contribution of patient costs in sub-Saharan Africa – a systematic review,” BMC Public Health 12 (2012), p. 980; K.N. Ukwaja, O. Modebe, C. Igwenyi, et al., “The economic burden of tuberculosis care for patients and households in Africa: A systematic review,” International Journal of Tuberculosis and Lung Disease 16/6 (2012), pp. 733-739.
81. World Health Organization (see note 13); World Health Organization (see note 39); Zachariah (see note 60); Akugizibwe (see note 61).
82. United Nations Committee on Economic Social and Cultural Rights (see note 52).
83. Munro (see note 15).
84. Division of Leprosy, Tuberculosis, and Lung Disease (see note 43).
85. Kizito (see note 69).
86. Hargreaves (see note 74).
87. M. Raviglione, B. Marais, K. Floyd, et al., “Scaling up interventions to achieve global tuberculosis control: progress and new developments,” Lancet 379/9829 (2012), pp. 1902-1913.
88. World Health Organization (see note 13); London (see note 23); World Health Organization (see note 39).
89. United Nations Committee on Economic Social and Cultural Rights (see note 52).
90. High Court of Kenya, Ngugi, M., Daniel Ng’etich v. Attorney General High Court of Kenya (Nairobi: High Court of Kenya, Republic of Kenya, 2016).
91. M. Ushio, “Amendment of tuberculosis prevention law and prospect of tuberculosis control program],” Kekkaku 80/8 (2005), pp. 541-546.
92. G.W. Mwai, G. Mburu, K. Torpey, et al., “Role and outcomes of community health workers in HIV care in sub-Saharan Africa: a systematic review,” Journal of the International AIDS Society 16 (2013), p. 18586.
93. World Care Council. The Patients’ Charter for Tuberculosis Care. (Paris: World Care Council, 2006). Available at http://www.who.int/tb/publications/2006/istc_charter.pdf
94. World Health Organization (see note 39).
95. Ibid.