João Biehl, Mariana P. Socal, Joseph J. Amon
Health and Human Rights 18/1
Published June 2016
Abstract
The impact of increasing numbers of lawsuits for access to medicines in Brazil is hotly debated. Government officials and scholars assert that the “judicialization of health” is driven by urban elites and private interests, and is used primarily to access high-cost drugs. Using a systematic sample of 1,262 lawsuits for access to medicines filed against the southern Brazilian state of Rio Grande do Sul, we assess these claims, offering empirical evidence that counters prevailing myths and affirms the heterogeneity of the judicialization phenomenon. Our findings show that the majority of patient-litigants are in fact poor and older individuals who do not live in major metropolitan areas and who depend on the state to provide their legal representation, and that the majority of medicines requested were already on governmental formularies. Our data challenge arguments that judicialization expands inequities and weakens the universal health care system. Our data also suggest that judicialization may serve as a grassroots instrument for the poor to hold the state accountable. Failing to acknowledge regional differences and attempting to fit all data into one singular narrative may be contributing to a biased interpretation of the nature of judicialization, and limiting the understanding of its drivers, consequences, and implications at local levels.
Introduction
“Judicialization increases health inequity.” So reads a recent headline in Folha de São Paulo, one of Brazil’s most influential newspapers.[1] The article highlights the growing phenomenon of lawsuits for access to medicines in the state of São Paulo, emphasizing that two-thirds of these lawsuits are filed by people with private health insurance or who attend private clinics, and concluding that they “originate from rich areas and concentrate on high-cost treatments.”[2]
As the justiciability of socioeconomic rights receives increasing interest internationally, the volume of individual right to health lawsuits in Brazil stands out.[3] Tens of thousands of cases are brought to Brazilian courts annually, the majority of which concern access to pharmaceuticals.[4] As the number of cases increases, there is growing controversy over the phenomenon and its consequences.
The article in Folha de São Paulo presents judicialization as a scandal of the “haves” triumphing over the “have-nots,” a view promoted by government officials and some public health scholars.[5] For example, Arthur Chioro, Brazil’s former health minister, said that lawsuits seeking medicines “take resources away from the poorest to benefit those who have more.”[6] David Uip, health secretary of the state of São Paulo, asserts: “It’s a kind of Robin Hood in reverse: to take from the poor to give to those who can afford to pay for a good lawyer.”[7] Álvaro Atallah, director of Brazil’s Cochrane Center, speculated that the pharmaceutical industry is behind the phenomenon of judicialization: “Why does no one file a lawsuit for the government to give calcium to pregnant women and prevent hypertension? Because calcium does not cost anything, there is no lobby behind it.”[8]
According to such narratives, judicialization is driven by urban elites and private interests and is used to access high-cost drugs that are not part of governmental formularies. It is reported that the people who file lawsuits are well-off litigants who are exploiting the expansiveness of the country’s constitutional right to health. Litigants are portrayed as undermining public health policies and furthering private-sector interests that constrain and deplete good government.
In this article, we present empirical evidence that challenges and refutes these anti-judicialization arguments. We contend that such arguments are part of a broader mythology of judicialization that ignores the complexity and regional heterogeneity of this phenomenon and ultimately misinforms public opinion and health policy.
Our conclusions derive from the analysis of a previously unpublished systematic sample of 1,262 lawsuits seeking access to medicines filed in 2008 against the southern Brazilian state of Rio Grande do Sul. With a population of 11 million people, Rio Grande do Sul has seen a sharp increase in health-related lawsuits in the past decade, rising from 1,126 new cases in 2002 to 17,025 new cases in 2009.[9] Roughly 70% of these lawsuits were for access to medicines.[10] By 2011, the state had the highest number of health-related lawsuits in the country, with 113,953 pending cases.[11]
Our findings demonstrate that the arguments decrying judicialization as a phenomenon that is driven by the rich, private lawyers, and pharmaceutical companies in order to access brand-name and high-cost medicines are, at least in Rio Grande do Sul, largely false. Our research found that right-to-health litigation in this state is a widespread practice that is utilized by low-income plaintiffs including the very poor. Rather than expanding inequities and weakening the universal health care system, judicialization may serve as a grassroots instrument for the poor to hold the government accountable for the planning and delivery of high-quality universal health coverage.
Methods
As part of a larger initiative aimed at understanding the judicialization of the right to health in Brazil, we created two databases of lawsuits seeking access to medicines from the state of Rio Grande do Sul. The results from our first database, a convenience sample of cases under review by the state solicitor general’s office, are described in previous articles published in The Lancet and Health and Human Rights Journal.[12] The second database is a representative sample of all lawsuits on access to medicines filed against the state in 2008, and is the basis for the analysis in this article. To create our second database, we accessed the Health Secretariat’s electronic registry that records all health-related lawsuits filed against the state. We drew a representative sample of all lawsuits requesting medicines by systematically collecting every sixth case of medicine-related lawsuits opened between January 1, 2008 and December 31, 2008, beginning with a randomly selected case. Lawsuits that, because of miscoding, did not represent a case seeking access to medicines were excluded.
Research assistants trained in law and pharmacy, and supervised by a physician and an attorney, reviewed data from the medicine-related lawsuits. Information on demographic characteristics of plaintiffs, their legal representation, medical diagnoses, the type and frequency of medicines requested, the legal arguments employed, and the immediate ruling of judges were excerpted.
Plaintiffs’ medical diagnoses were classified according to the 10th edition of the International Classification of Diseases.[13] Requested medicines were classified according to the drug formularies of Brazil’s public health care system: 1) low-cost “essential medicines” provided by municipal administrations, 2) high-cost “exceptional medicines” funded by the federal government and distributed by states to treat select diseases according to national therapeutic guidelines (this formulary was since renamed the “Specialized Component of Pharmaceutical Assistance”), and 3) “special medicines” provided by individual states to attend to the specific needs of their populations.[14]
The procedures for patients to access publicly funded medicines vary depending on the type of medicine sought and how it is categorized by the government. In order to obtain essential medicines, patients must present a prescription at a local public pharmacy. In order to obtain medicines from the special and exceptional drug formularies, patients must submit proof of medical need and file an administrative request. For most of these high-cost medicines, patients must submit additional medical information demonstrating that the request is in accordance with national therapeutic guidelines. We use the terms “on-formulary” and “off-formulary” to refer to medicines that were or were not included in governmental drug formularies at the time of data collection.
To determine monthly treatment costs, we examined costs from two sources: the total monthly cost of all medicines sought as reported by plaintiffs in the lawsuits, and the monthly cost of individual medicines according to the Brazilian Ministry of Health database.[15] While plaintiffs presenting health-related lawsuits are required to estimate costs based on the average price of medicines from three independent pharmacies, the Health Secretariat’s electronic registry provides only an aggregate total of the cost of all medicines sought. By contrast, the Ministry of Health’s database provides the government-negotiated cost of individual medicines, which are likely to be lower than the prices from private pharmacies.
Data was entered in Microsoft Access and regular checks were conducted by senior researchers for data quality and completeness. Data was analyzed using Stata Statistical Software for variable frequency, distribution, and cross-tabulations, including chi-square tests of significance.[16]
The research was reviewed and approved by the Institutional Review Board of Princeton University. The research was also approved by the Health Secretariat and the Solicitor General’s Office of the state of Rio Grande do Sul, the latter of which also established guidelines for data collection that guaranteed the confidentiality of medical and legal information. For this research, only de-identified data were analyzed and informed consent from the plaintiffs was not sought.
Results
Of 8,559 medicine-seeking lawsuits filed in 2008 against the state of Rio Grande do Sul, 1,404 cases were selected. One hundred and forty-two cases were excluded as ineligible, leaving a total of 1,262 cases that were included in our database. Among the individual patient-plaintiffs, 54% (n=685) were female and 40% (n=510) were married. Plaintiffs reported suffering from an average of 1.38 diseases (range: 1-7) and sought an average of 2.75 medicines (range: 1-23) per lawsuit, the majority (79%, n=998) for continuous use (see Table 1). In what follows, we describe these and other results in detail and present them as counter-evidence to prevailing myths.
Myth 1: Judicialization is driven by urban elites and is not available to the poor.[17]
Our study found that judicialization is widespread in both metropolitan and rural areas, and that individuals who filed lawsuits seeking access to medicines were predominantly of lower socioeconomic status. The majority of plaintiffs were adults (61%) or elderly (24%). The overwhelming majority of cases (92%, n=1160) came from outside the state capital. Around half of the plaintiffs were either retired (32%) or unemployed (21%). Manual or service sector workers, including farming and domestic workers, made up 15% of the sample. Less than 5% of the plaintiffs were professionals, or administrative or technical workers (see Table 1).
Although we did not have access to data on income, the plaintiffs’ legal representation offers indirect evidence of their economic status. Within our sample, more than half of the plaintiffs were represented by the Public Defender’s Office, which, according to Brazilian law, provides free legal assistance to people classified as low-income (defined as earning three times the national minimum wage or less). Brazilian law also allows for individuals without the ability to pay to request that the state pay legal fees. In 91% of the lawsuits (n=1,147) plaintiffs requested this support.
Myth 2: Judicialization is driven by private attorneys specializing in health-related lawsuits and physicians seeking to promote high-cost treatments.[18]
Our study found that judicialization is mostly driven by patients represented by public attorneys, including the Public Defender’s Office (57% (n=724)); the Federal Legal Counsel, which represents children under the age of 10, as well as other vulnerable and minority groups (7% (n=89)); and university law clinics (2% (n=30)). In contrast, private lawyers represented about one-third of plaintiffs (32%, n=407) (see Table 1).
A total of 311 different private attorneys represented 407 plaintiffs, averaging 1.3 lawsuits per attorney. Eighty-six percent (n=267) of all lawyers represented only one plaintiff. Thirty-four lawyers (11%) represented two plaintiffs, and two lawyers (0.6%) represented three plaintiffs. Seven lawyers (1.8%) represented more than three plaintiffs, one of whom represented 13 plaintiffs and the other, 23.
In the 23 lawsuits represented by a single lawyer, the medicines most often requested (in 6 of the 23 lawsuits) were two asthma medications that, according to governmental drug formularies, should already be available in the public health system. Only one lawsuit represented by this attorney sought access to a high-cost treatment: adalimumab (US$ 5,677.63 per month), a medicine to treat rheumatoid arthritis that was also part of the government drug formulary.
The lawyer representing the second highest number of plaintiffs had a different pattern: 11 out of the 13 lawsuits he presented requested food supplements used to treat malabsorption, celiac disease, lactose intolerance, and allergic colitis and/or gastroenteritis.
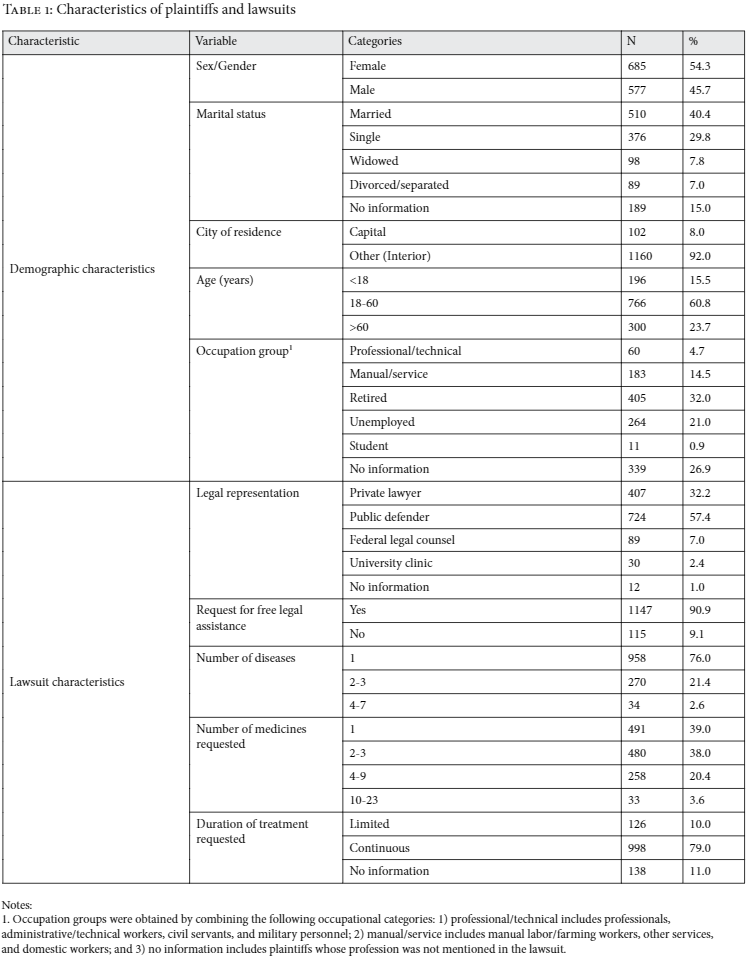
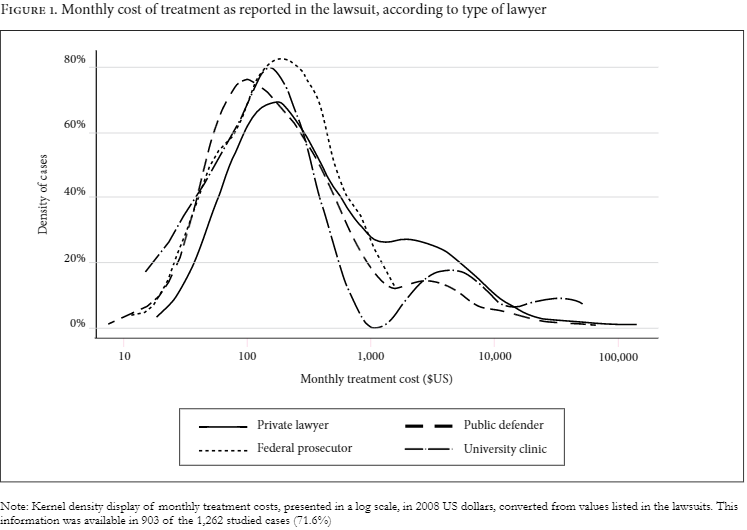
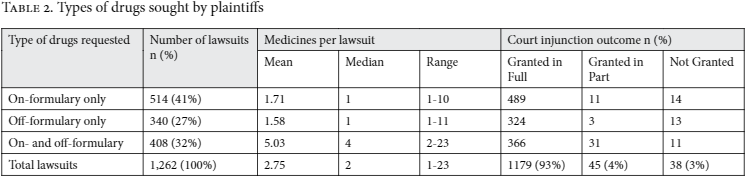
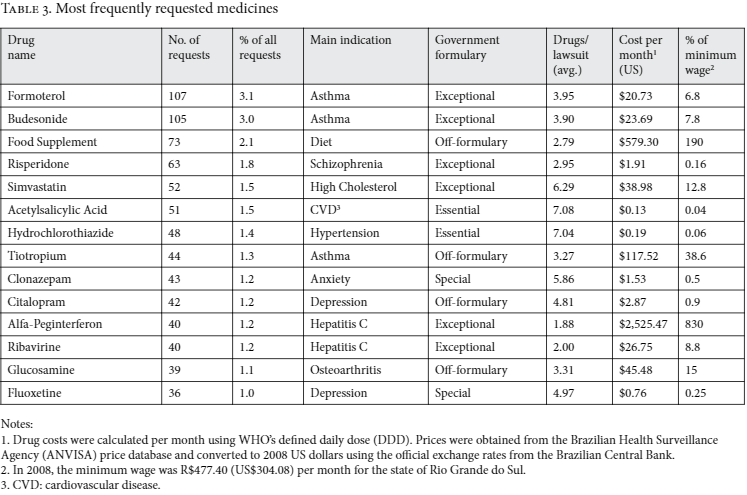
There was no difference between public and private attorneys in terms of whether the medicines requested were on- or off-government formularies, nor to which specific formulary they belonged (chi-square test, p=0.735). The distribution of monthly treatment cost, among lawsuits according to type of lawyer, was broadly similar (see Figure 1).
Note: Monthly treatment costs are presented in 2008 US dollars, converted from values listed in the lawsuits. This information was available in 903 of the 1,262 studied cases (71.6%).
As with private attorneys, our study found no evidence that judicialization was driven by a small number of physicians seeking to promote specialized or high-cost treatments. The ratios of lawsuits per doctor were similar to the ratios of lawsuits per lawyer. We identified 851 physicians who provided medical evidence (prescriptions or reports) in the 1,262 lawsuits of our sample, with an average of 1.26 cases per physician. The majority of physicians (80.4%, n=684) appeared in only one case. Fifteen percent of physicians (n=131) appeared in two cases and 2.4% of physicians (n=20) appeared in three cases. A total of 14 physicians appeared in four or more cases (1.6%), and one of them appeared in nine cases. The prescription patterns of the 14 physicians with four or more cases reflected their fields of medical specialization and the medicines prescribed to patients were diverse.
Myth 3: Judicialization is mostly used to access high-cost and off-formulary drugs.[19]
Our study found that the majority of patients requested low-cost drugs that were part of governmental drug formularies and that should have been publicly available. A total of 3,468 medicines were requested in the 1,262 lawsuits. Over half of the requested medicines (n=1,940, 56%) were part of governmental drug formularies: 18% (n=609) belonged to the essential medicines formulary, 9% (n=309) to the special medicines formulary, and 29% (n=1,022) to the exceptional medicines formulary. Overall, 73% of all plaintiffs requested at least one medicine that was part of governmental drug formularies. Forty-one percent of the plaintiffs requested on-formulary medicines exclusively, while 27% requested off-formulary medicines exclusively (see Table 2).
Table 3 shows the 14 most frequently requested medicines, which accounted for 22.6% of all requests. Eleven of the 14 were part of governmental drug formularies and several had therapeutic guidelines defined by the Ministry of Health. These medicines were mainly indicated to treat medical conditions highly prevalent in the state, including asthma, high cholesterol, and hypertension. The monthly price of these medicines ranged from US$0.13 to US$2,525. Only 2 of the 14 most commonly requested medicines had monthly costs higher than the minimum wage for one month (US$304).Using information available in the lawsuits, the plaintiff-reported median monthly cost of treatment was US$185 (range: US$10–US$89,172). Median monthly cost varied little between lawsuits requesting on-formulary only medicines (US$176), off-formulary only (US$192), or both on- and off-formulary (US$184). Overall, a minority of lawsuits drove most of the costs: 1.6% percent of the lawsuits (20 cases) had monthly costs above US$10,000 and 0.8% percent (10 cases) had monthly costs above US$30,000.
1 Drug costs were calculated per month using WHO’s defined daily dose (DDD). Prices were obtained from the Brazilian Health Surveillance Agency (ANVISA) price database and converted to 2008 US dollars using the official exchange rates from the Brazilian Central Bank. Notes:
2. In 2008, the minimum wage was R$477.40 (US$304.08) per month for the state of Rio Grande do Sul.
3. CVD: cardiovascular disease.
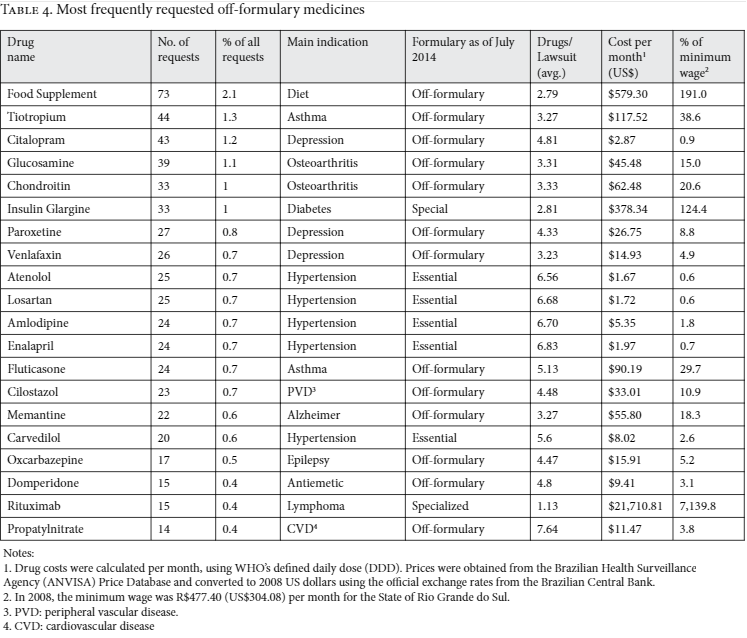
Table 4 shows the 20 most frequently requested medicines that were not part of governmental drug formularies. Most of these medicines are indicated to treat common medical conditions such as depression, hypertension, and arthritis. The monthly price of these medicines ranged from US$1.67 to US$21,711.
Myth 4: Judicialization disrupts health policy-making and bypasses administrative procedures designed for appropriate, efficient, and equitable access to medicines.[20]
Just as the majority of plaintiffs requested medicines that were part of governmental drug formularies, our study also found that the majority of plaintiffs initially tried to secure their treatments through local administrative channels, and that they provided proof of medical need once they brought their claims to court.
Of the 820 individuals in our sample whose lawsuits requested medicines from special or exceptional drug formularies, 481 (68.7%) had followed administrative procedures and had previously requested these medicines through local or regional health services. Of the 1,262 lawsuits examined, 1,076 lawsuits (85.3%) included a physician-issued prescription or a report confirming the need for treatment.
In the overwhelming majority of cases, district judges ruled in favor of patients. In 93.4% (n=1,179) of all lawsuits, district judges granted plaintiffs an immediate injunction in full for access to the requested medicines and in 3.6% (n=45) of cases, they granted a partial injunction. Three percent of plaintiffs (n=38) had their claims denied (see Table 2). Among these cases, 29 had information on the judge’s reason for the denial. In 23 cases, the plaintiffs were requesting drugs that were either experimental (n=4) or not on government formularies (n=19), and in three cases, judges ruled that plaintiffs did not follow proper administrative procedures.
To investigate whether judges took governmental guidelines for drug provision into consideration, we analyzed the frequency with which judges ruled on the provision of generics. In the 837 rulings in which there was explicit reference to the drug form, judges mandated the provision of generics in 75.9% (n=635) of cases and mandated the provision of a brand-name drug in 24.1% (n=202) of cases.
Concerned with the question of whether judicialization leads to policy reforms, we analyzed whether the 329 off-formulary medicines that had been requested by the plaintiffs in 2008 were subsequently introduced into the public health system. By 2014, 59 (17.9%) of these medicines had been incorporated into the governmental formularies: 35 in the essential medicines formulary, 12 in the exceptional medicines formulary (by then renamed the “specialized component” formulary), and three in the special medicines formulary. The remaining nine off-formulary medicines were incorporated into governmental disease-specific strategic programs. Among the 20 most frequently requested off-formulary medicines in our sample, seven have been incorporated into governmental formularies since 2008, including the most expensive one (see Table 4).
Discussion
Government officials, scholars, and journalists have repeatedly asserted that the increasing number of lawsuits seeking access to medicines in Brazil is a phenomenon of elites that subverts efficient health governance and produces inequality.[21] In our view, this line of criticism, at least in Rio Grande do Sul, is inaccurate and does not account for the on-the-ground realities of patient-plaintiffs. Nor does it acknowledge the political possibility that individual litigation represents. The judicialization of the right to health in Brazil is not a single phenomenon, and failing to acknowledge regional differences and attempting to fit all data into one singular narrative may be contributing to a biased interpretation of the nature of judicialization, and limiting the understanding of its drivers, consequences, and implications at local levels.
The results of our study – which includes the largest number of lawsuits seeking access to medicine in Brazil to date – reveal a process of judicialization from below, stemming from poor and older individuals who do not live in major metropolitan areas, and who depend on the state to provide their legal representation. We did not find that judicialization represented a phenomenon of “Robin Hood in reverse”; quite the contrary: we found evidence that judicialization largely serves the disadvantaged who turn to the courts to secure a wide range of medicines, more than half of which are on government formularies and should be available in government health centers.[22]
Within the debate over judicialization, critics have often called for more attention to economic analyses and evidence-based medicine. Yet judicialization can itself help to create an alternative source of information: practice-based evidence. Practice-based evidence points to where existing administrative mechanisms fail people and offers clues on how to improve the management of public health. Moreover, although often dismissed as such, individual demands are not simply the antithesis of collective need; individual experiences are shaped by common phenomena within different communities. Right to health litigation is not a perfect process—it is costly administratively and in the toll it takes on individuals and their families. But it is also a valuable opportunity for citizens’ diverse and often urgent (life or death) demands to be brought to the attention of the state. Certainly, litigation is not a substitute for health policy, but it can be a crucial adjunct.[23] Individual claims can highlight gaps in health planning, policy, and delivery, as well the lack of responsiveness of health systems to the citizens they aim to serve.
The debate over judicialization reflects deeper tensions over the state’s obligations and challenges in fulfilling the right to health as enshrined in Brazil’s 1988 constitution. The constitution mandated the creation of the national health care system to guarantee health as “a right of all and a duty of the state.”[24] While the constitution identified core characteristics and values for the delivery of health care, such as universality, comprehensiveness, and equity, it did not define the specific content of the right to health or the boundaries of universal health care. In practice, it was implemented without transparent criteria, the full participation of patients or civil society, or solid mechanisms to ensure future reform and innovation. Lacking clear limits for which interventions should be included in the public benefits package, the state relied on ad-hoc decision-making while patients sought the expansion of benefits via the courts. Judges interpreted the constitutional mandate expansively and rejected state arguments that the available resources for health expenditures were exhausted.
In addressing a dysfunctional health system that fails to provide for their needs, low-income patients face the option of exiting the public system (seeking private sector alternatives), or voicing concerns through cumbersome and slow political and participatory mechanisms like voting or community councils. Our study shows that through right to health litigation, some Brazilian citizens are finding new ways of concretizing voice through a process of entering justice, acting as political subjects to hold the state accountable, and exposing the realpolitik of executive and legislative bodies.[25] As subjects who cannot resort to the health market but expect, as citizens, that the state care for them, they are using judicialization to simultaneously demand services and to make the system respond to their expressed needs and its own failures.
Our study also showed that when patients sought new technologies not yet included in (obsolete) benefits packages, the state tended to incorporate the requested technologies into official policies posteriorly. While in no way a magic bullet for broader structural political problems, judicialization can thus be understood as a crucial mechanism of both accountability and responsiveness, highlighting gaps in the system and sometimes (however modestly) addressing them.
It is possible that the presence of strong, accessible, and widespread public institutions such as the Public Defender’s Office act as important enablers of judicialization. Rio Grande do Sul has a much higher volume of right-to-health litigation than other Brazilian states, with more cases than the next four states with the most litigation (São Paulo, Rio de Janeiro, Ceará, and Minas Gerais, respectively) combined. These differences reflect the varied performance of the decentralized health care system throughout the country, as well as the significant differences in economy, demography, and administrative capacity within and across the 26 Brazilian states. Our research suggests that there may be a relationship between stronger public institutions and more intense judicialization and, thus, while judicialization may reduce inequalities within a state, it might have little impact on inequalities across states. At the very least, the heterogeneity of right-to-health litigation across the Brazilian states indicates the need for a more nuanced and in-depth analysis of its drivers and implications at local levels.
Conclusion
Our study challenged myths about the negative impact of judicialization on both public health administration and on the broader question of equitable access to care in Brazil. While directly based on work in the south of Brazil, the information presented here is also relevant to national and international discussions of how to advance the goal of universal health coverage.[26]
Countries have legitimate concerns about regulating new and high-cost medicines, and resource constraints mean that trade-offs will inevitably occur. More broadly, countries face difficult decisions about allocating funding for pharmaceuticals or towards targeting the social determinants of health, especially within contexts of aging populations, increasing life expectancies, and the rise of non-communicable diseases.[27] Grappling with such questions will require people-centered systems and mechanisms for responsiveness and change, issues that have not yet been sufficiently explored in current debates over judicialization and universal health coverage.
Brazil’s experience highlights the importance of ensuring explicit and functional mechanisms for participation, transparency, and accountability when launching universal health care. It also illustrates the significant role of civil society and the judiciary in monitoring the quality of health care and assessing the need for new medical technologies amidst competing and contested considerations of value, cost-effectiveness, and efficiency. The role of the judiciary in this regard is uneven within the region more generally, and some scholars have argued that courts are better suited to enforcing access to agreed upon care than in promoting rational and equitable priority-setting for the health sector.[28] However, our study shows the importance of judicialization as a mechanism for state accountability in driving advancements towards quality universal health coverage and transparent and participatory priority-setting.
Acknowledgments
The authors acknowledge the research support of the Ford Foundation and of Princeton University’s Health Grand Challenges Initiative. These institutions had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
João Biehl, PhD, is the Susan Dod Brown Professor of Anthropology and Woodrow Wilson School Faculty Associate, Princeton University, Princeton, USA.
Mariana P. Socal, MD, MSc, MPP, is a Doctoral Candidate at the Johns Hopkins Bloomberg School of Public Health, Baltimore, USA.
Joseph J. Amon, PhD, MSPH, is a Visiting Lecturer of Public and International Affairs at the Woodrow Wilson School, Princeton University, Princeton, USA.
Please address correspondence to João Biehl: jbiehl@princeton.edu.
References
- C. Colucci, “Judicialização Faz Desigualdade na Saúde Avançar,” Folha de São Paulo (Mar 29, 2014). Available at http://www1.folha.uol.com.br/fsp/especial/158639-judicializacao-faz-desigualdade-na-saude-avancar.shtml.
- Ibid.
- V. Gauri and D.M. Brinks, Courting Social Justice: Judicial Enforcement of Social and Economic Rights in the Developing World (Cambridge, UK: Cambridge University Press, 2008); A.E. Yamin and S. Gloppen, Litigating Health Rights: Can Courts Bring More Justice to Health? (Cambridge, MA: Harvard University Press, 2011).
- J. Biehl, J.J. Amon, M.P. Socal, and A. Petryna, “Between the court and the clinic: lawsuits for medicines and the right to health in Brazil,” Health Hum Rights 14 (2012), pp. 36–52; J. Biehl, A. Petryna, A. Gertner, et al., “Judicialisation of the right to health in Brazil,” Lancet 373 (2009), pp. 2182–2184.
- S. Azevedo, “Remédios nos Tribunais,” Revista Época (Dec 12, 2007). Available at http://revistaepoca.globo.com/Revista/Epoca/0,,ERT59499-15257,00.html; A.L. Chieffi and R.B. Barata, “Judicialization’ of public health policy for distribution of medicines,” Cad Saude Publica 25 (2009), pp.1839–1849; V.A. Da Silva and F.V. Terrazas, “Claiming the Right to Health in Brazilian Courts: The Exclusion of the Already Excluded,” The Social Sciences Research Network (May 15, 2008). Available at http://ssrn.com/abstract=1133620; Economic Intelligence Unit, “Broadening Health Care Access in Brazil through Innovation” (2010), Available at http://www.economistinsights.com/healthcare/analysis/broadening-healthcare-access-brazil-through-innovation; The Economist, “An Injection of Reality” (Jul 30, 2011) The Economist. Available at http://www.economist.com/node/21524879; O.L. Ferraz, “The right to health in the courts of Brazil: worsening health inequities?” Health and Human Rights 11 (2009), pp.33–45; V.L. Pepe, M. Ventura, J.M. Sant’ana, et al., “Characterization of lawsuits for the supply of “essential” medicines in the State of Rio de Janeiro, Brazil,” Cad Saude Publica 26 (2010), pp. 461–471; F.S. Vieira and P. Zucchi, “Distortions to national drug policy caused by lawsuits in Brazil,” Rev Saude Publica 41(2007), pp. 214–222.
- Colucci (see note 1).
- Ibid.
- Ibid.
- Biehl et al. (2012, see note 4).
- Biehl et al. (2009, see note 4).
- F. Bassette, “RS reúne metade das ações judiciais de saúde.” O Estado de São Paulo (Apr 29, 2011), Available at http://www.estadao.com.br/noticias/geral,rs-reune-metade-das-acoes-judiciais-de-saude-imp-,712418.
- Biehl et al. (2012, see note 4).
- World Health Organization. International Classification of Diseases-10 (ICD-10). (WHO, 2015). Available at: http://www.who.int/classifications/icd/en/.
- Ministry of Health, “Protocolos Clínicos e Diretrizes Terapêuticas” (Brasília, DF: Ministério da Saúde, 2010).
- Ministry of Health, “Banco de Preços em Saúde.” (2014). Available at http://portalsaude.saude.gov.br/index.php/cidadao/principal/banco-de-precos-em-saude/mais-banco-de-precos-em-saude.
- Microsoft Access®, Microsoft Corporation, Redmond, WA, Stata Statistical Software: Release 12, (College Station, TX: StataCorp LP, 2011).
- D.C. Borges and M.A. Uga, “Conflicts and impasses in the judicialization of the supply of medicines: circuit court rulings on claims brought against the State of Rio de Janeiro, Brazil, in 2005,” Cad Saude Publica 26 (2010), pp. 59–69; Chieffi and Barata (2009, see note 5); Colucci (see note 1); Da Silva and Terrazas (see note 5); Ferraz (see note 5); C.G. Victora, M.L. Barreto, M. do Carmo Leal, et al, “Health conditions and health-policy innovations in Brazil: the way forward,” Lancet 377 (2011), pp. 2042–2053.
- O.H. Campos Neto, F. de A. Acurcio, M.A. Machado, et al., “Doctors, lawyers and pharmaceutical industry on health lawsuits in Minas Gerais, Southeastern Brazil.” Rev Saude Publica 46 (2012), pp. 784–790; Chieffi and Barata (2009, see note 5); A.L. Chieffi and R.C. Barata, “Legal suits: pharmaceutical industry strategies to introduce new drugs in the Brazilian public healthcare system.” Rev Saude Publica 44 (2010), pp. 421–428; Victoria et al (see note 17); D.W.L. Wang and O.L. Ferraz, “Reaching Out to the Needy? Access to Justice and Public Attorneys’ Role in Right to Health Litigation in the City of São Paulo,” SUR International Journal on Human Rights 10/18 (2013), pp. 159-179.
- Borges and Uga (see note 17); Chieffi and Barata (2009, see note 5); Da Silva and Terrazas (see note 5); C. Iriart, T. Franco, and E.E. Merhy, “The creation of the health consumer: challenges on health sector regulation after managed care era,” Globalization and Health 7 (2011), p. 2; L.C. Lopes, S. Barberato-Filho, A.C. Costa, and C.G. Osorio-de-Castro, “Rational use of anticancer drugs and patient lawsuits in the state of Sao Paulo, Southeastern Brazil,” Rev Saude Publica 44 (2010), pp. 620–628; Vieira and Zucchi (see note 5).
- Borges and Uga (see note 17); Chieffi and Barata (2009, see note 5); D. Diniz, M. Medeiros, and I.V. Schwartz, “Consequences of the judicialization of health policies: the cost of medicines for mucopolysaccharidosis,” Cad Saude Publica 28 (2012), pp. 479–489; Economic Intelligence Unit (see note 5); V.S. Gomes and T.A. Amador, “Studies published in indexed journals on lawsuits for medicines in Brazil: a systematic review,” Cad Saude Publica 31/3 (2015), pp. 451-62; Government of Brazil, “Constituição da República Federativa do Brasil de 1988.” (1988), available at http://www.planalto.gov.br/ccivil_03/constituicao/constituicao.htm; J. Luo, M.A. Oliveira, M.B. Ramos, et al., “Antiretroviral drug expenditure, pricing and judicial demand: an analysis of federal procurement data in Brazil from 2004-2011.” BMC Public Health 14 (2014), p. 367; M.A. Machado, F. de A. Acurcio, C.M. Brandão, et al., “Judicialization of access to medicines in Minas Gerais state, Southeastern Brazil.” Rev Saude Publica 45(2011), pp. 590–598; S.B. Marques and S.G. Dallari, “Safeguarding of the social right to pharmaceutical assistance in the state of São Paulo, Brazil,” Rev Saude Publica 41 (2007), pp.101–107; M. Medeiros, D. Diniz and I.V. Schwartz, “The thesis of judicialization of health care by the elites: medication for mucopolysaccharidosis,” Cien Saude Colet 18 (2013), pp. 1079–1088; A.M. Messeder, C.G. Osorio-de-Castro, and V.L. Luiza, “Can court injunctions guarantee access to medicines in the public sector? The experience in the State of Rio de Janeiro, Brazil,” Cad Saude Publica 21 (2005), pp. 525–534; J.M. Sant’ana, V.L. Pepe, T.A. Figueiredo, et al., “Rational therapeutics: health-related elements in lawsuits demanding medicines.” Rev Saude Publica 45 (2011), pp. 714–721; J.D. Sartori, P.G. Leivas, M.V. Souza, et al., “Court-ordered access to treatment of rare genetic diseases: Fabry Disease in the state of Rio Grande do Sul, Brazil.” Cien Saude Colet 17 (2012), pp. 2717–2728; Vieira and Zucchi (see note 5); D.W.L. Wang, “Can litigation promote fairness in healthcare? The judicial review of rationing decisions in Brazil and England.” (London, UK: The London School of Economics and Political Science, 2013); A.E. Yamin, “Promoting Equity in Health: What Role for Courts?” Health Hum Rights 16/2) (2014), pp. 1-9.
- Chieffi and Barata (2010; see note 18); Colucci (see note 1); Da Silva and Terrazas (see note 5); Gomes and Amador (see note 17); Lopes et al (see note 19); Vieira and Zucchi (see note 5); Wang (see note 20); Wang and Ferraz (see note 18).
- Biehl et al. (2012, see note 4).
- Yamin (see note 20).
- Government of Brazil (see note 20),
- A. Hirschman, Exit, Voice, and Loyalty: Responses to Decline in Firms, Organizations, and States (Cambridge, MA: Harvard University Press, 1970).
- R. Dittrich, L. Cubillos, L. Gostin et al., “The International Right to Health: What Does It Mean in Legal Practice and How Can It Affect Priority Setting for Universal Health Coverage?” Health Systems & Reform 2/1 (2016), pp. 23-31; L. Reveiz, E. Chapman, R. Torres, J. F. Fitzgerald, A. Mendoza, M. Bolis, et al., “Litigios por derecho a la salud en tres países de América Latina: revisión sistemática de la literature,” Rev Panam Salud Publica 33 (2013), pp. 213–22.
- R. Iunes, L. Cubillos-Turriago, and M.L. Escobar, “Universal Health Coverage and Litigation in Latin America,” En Breve 178 (2012), pp. 1-4.
- Dittrich et al. (see note 26); O. F. Nordheim and S. Gloppen, “Litigating for Medicines: How Can We Assess Impact on Health Outcomes?” In: A.E. Yamin and S. Gloppen, Litigating Health Rights: Can Courts Bring More Justice to Health? (Cambridge, MA: Harvard University Press, 2011), pp. 304-332.